Abstract
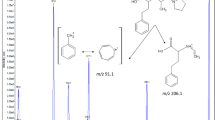
(S)-2-ethoxy-3-(4-{3-methyl-5-[4-(3-methyl-isoxazol-5-yl)-phenyl]thiophen-2-ylmethoxy}-phenyl)-propionic acid (PAM-1616) is a novel peroxisome proliferators-activated receptor γ (PPARγ) partial agonist with excellent antihyperglycemic activity. It is a promising new drug candidate for the treatment of type-2 diabetes with reduced possibility of edema in vitro/in vivo. In order to evaluate the pharmacokinetics of PAM-1616, a reliable, selective and sensitive highperformance liquid chromatography/electrospray ionization tandem mass spectrometry was developed for the quantification of PAM-1616 in rat plasma. The analytes were extracted from rat plasma with ethyl acetate, separated on an Atlantis dC18 column with a mobile phase of 75% acetonitrile in 10 mM ammonium formate (pH 4.5), and detected by tandem mass spectrometry in the selective reaction monitoring mode. The calibration curve was linear (r 2 = 0.999) over the concentration range of 0.05–20.0 μg/mL and the lower limit of quantification was 0.05 μg/mL. The coefficient of variation and relative error at four QC levels were 1.8% to 14.3% and −10.0% to 6.5%, respectively. The present method was successfully applied to the pharmacokinetic study of PAM-1616 after intravenous administration of PAM-1616 potassium at a dose of 1 mg/kg in rats.
Similar content being viewed by others

References
Berger, J. P., Petro, A. E., Macnaul, K. L., Kelly, L. J., Zhang, B. B., Richards, K., Elbrecht, A., Johnson, B. A., Zhou, G., Doebber, T. W., Biswas, C., Parikh, M., Sharma, N., Tanen, M. R., Thompson, G. M., Ventre, J., Adams, A. D., Mosley, R., Surwit, R. S., and Moller, D. E., Distinct properties and advantages of a novel peroxisome proliferator-activated protein γ selective modulator. Mol. Endocrinol., 17, 662–676 (2003).
Berger, J. P., Akiyama, T. E., and Meinke, P. T., PPARs: therapeutic targets for metabolic disease. Trends Pharmacol. Sci., 26, 244–251 (2005).
Gurnell, M., Savage, D. B., Chatterjee, V. K., and O’Rahilly, S., The metabolic syndrome: Peroxisome proliferator-activated receptor gamma and its therapeutic modulation. J. Clin. Endocrinol. Metab., 88, 2412–2421 (2003).
Hsieh, Y., HPLC-MS/MS in drug metabolism and pharmacokinetic screening. Expert Opin. Drug Metab. Toxicol., 4, 93–101 (2008).
Kim, D. K., Park, E. J., Jeong, J. H., Jeon, S. H., Kim, E. J., Shim, H. J., Lim, J. I., and Lee, H. S., Liquid chromatography-tandem mass spectrometry of a new PPARα/γ dual agonist PAR-5359 in rat plasma. Arch. Pharm. Res., 32, 1743–1748 (2009).
Kim, S. J., Lee, S. S., Ji, H. Y., Lee, H. I., Lee, S., Yi, K. Y., Yoo, S. E., Hwang, J., and Lee, H. S., Determination of a novel antiangiogenic agent KR-31831 in rat plasma by liquid chromatography-tandem mass spectrometry. Anal. Lett., 37, 283–292 (2004).
Korfmacher, W. A., Principles and applications of LC-MS in new drug discovery. Drug Discov. Today, 10, 1357–1367 (2005).
Larsen, P. J., Lykkegaard, K., Larsen, L. K., Fleckner, J., Sauerberg, P., Wassermann, K., and Wulff, E. M., Dissociation of antihyperglycaemic and adverse effects of partial perioxisome proliferator-activated receptor (PPAR-γ) agonist balaglitazone. Eur. J. Pharmacol., 596, 173–179 (2008).
Nesto, R. W., Bell, D., Bonow, R. O., Fonseca, V., Grundy, S. M., Horton, E. S., Le Winter, M., Porte, D., Semenkovich, C. F., Smith, S., Young, L. H., and Kahn, R., Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Circulation, 108, 2941–2948 (2003).
Olefsky, J. M., Treatment of insulin resistance with peroxisome proliferator-activated receptor gamma agonists. J. Clin. Invest., 106, 467–472 (2000).
Paek, I. B., Ji, H. Y., Kim, M. S., Lee, G., and Lee, H. S., Metabolism of a new P-glycoprotein inhibitor HM-30181 in rats using liquid chromatography/electrospray mass spectrometry. Rapid Commun. Mass Spectrom., 20, 1457–1462 (2006).
Picard, F. and Auwerx, J., PPARγ and glucose homeostasis. Annu. Rev. Nutr., 22, 167–197 (2002).
White, R. E., High-throughput screening in drug metabolism and pharmacokinetic support of drug discovery. Annu. Rev. Pharmacol. Toxicol., 40, 133–157 (2000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chae, H.W., Jung, H., Kim, E.J. et al. Quantification of a novel PPARγ partial agonist (S)-2-ethoxy-3-(4-{3-methyl-5-[4-(3-methyl-isoxazol-5-yl)-phenyl]thiophen-2-ylmethoxy}-phenyl)-propionic acid (PAM-1616) in rat plasma using liquid chromatography-tandem mass spectrometry. Arch. Pharm. Res. 33, 1389–1394 (2010). https://doi.org/10.1007/s12272-010-0912-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-010-0912-z