Abstract
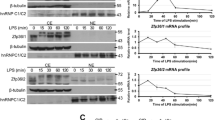
Phenylarsine oxide (PAO), a membrane-permeable trivalent arsenical, is widely used as an inhibitor of protein tyrosine phosphatases. It reacts with vicinal sulfhydryl groups of proteins to form stable ring structures. Here we show the regulatory function of PAO in immune responses from macrophages. PAO significantly induced the secretion of interleukin (IL)-12p40 in lipopolysaccharide-stimulated macrophages. The mRNA expression and the gene promoter activity of IL-12p40 were enhanced by PAO. These results suggest that PAO may enhance IL-12p40 production at the transcriptional level. Furthermore, the effects of PAO on several signaling molecules regulating IL-12p40 expression were investigated. PAO attenuated the induced binding activity of cAMP response element (CRE), but not of NF-κB. Moreover, CRE promoter activity was dose-dependently inhibited by PAO and the increased secretion of IL-12p40 by PAO was reduced by forskolin, a cAMP activator. These results suggest that PAO enhances IL-12p40 production by inhibiting CRE activity.
Similar content being viewed by others
References
Becker, C., Wirtz, S., Ma, X., Blessing, M., Galle, P. R., and Neurath, M. F., Regulation of IL-12 p40 promoter activity in primary human monocytes: roles of NF-kappaB, CCAAT/enhancer-binding protein beta, and PU.1 and identification of a novel repressor element (GA-12) that responds to IL-4 and prostaglandin E(2). J. Immunol., 167, 2608–2618 (2001).
Frearson, J. A. and Alexander, D. R., The phosphotyrosine phosphatase SHP-2 participates in a multimeric signaling complex and regulates T cell receptor (TCR) coupling to the Ras/mitogen-activated protein kinase (MAPK) pathway in Jurkat T cells. J. Exp. Med., 187, 1417–1426 (1998).
Hasko, G. and Szabo, C., IL-12 as a therapeutic target for pharmacological modulation in immune-mediated and inflammatory diseases: regulation of T helper 1/T helper 2 responses. Br. J. Pharmacol., 127, 1295–1304 (1999).
Hmadcha, A., Carballo, M., Conde, M., Marquez, G., Monteseirin, J., Martin-nieto, J., Bedoya, F. J., Pintado, E., and Sobrino, F., Phenylarsine oxide increases intracellular calcium mobility and inhibits Ca(2+)-dependent ATPase activity in thymocytes. Mol. Genet. Metab., 68, 363–370 (1999).
Iwanaga, K., Yang Y., Raso, M. G., Ma, L., Hanna, A. E., Thilaganathan, N., Moghaddam, S., Evans, C. M., Li, H., Cai, W. W., Sato, M., Minna, J. D., Wu, H., Creighton, C. J., Demayo, F. J., Wistuba, II, and Kurie, J. M., Pten inactivation accelerates oncogenic K-ras-initiated tumorigenesis in a mouse model of lung cancer. Cancer Res., 68, 1119–1127 (2008).
Kalef, E., Walfish, P. G., and Gitler, C., Arsenical-based affinity chromatography of vicinal dithiol-containing proteins: purification of L1210 leukemia cytoplasmic proteins and the recombinant rat c-erb A beta 1 T3 receptor. Anal. Biochem., 212, 325–334 (1993).
Kamata, T., Yamashita, M., Kimura, M., Murata, K., Inami, M., Shimizu C., Sugaya, K., Wang, C. R., Taniguchi, M., and Nakayama, T., src homology 2 domain-containing tyrosine phosphatase SHP-1 controls the development of allergic airway inflammation. J. Clin. Invest., 111, 109–119 (2003).
Kang, B. Y. and Kim, T. S., Targeting cytokines of the interleukin-12 family in autoimmunity. Curr. Med. Chem., 13, 1149–1156 (2006).
Kim, L., Del rio, L., Butcher, B. A., Mogensen, T. H., Paludan, S. R., Flavell, R. A., and Denkers, E. Y., p38 MAPK autophosphorylation drives macrophage IL-12 production during intracellular infection. J. Immunol., 174, 4178–4184 (2005).
Kurita-taniguchi, M., Fukui, A., Hazeki, K., Hirano, A., Tsuji, S., Matsumoto, M., Watanabe, M., Ueda, S., and Seya, T., Functional modulation of human macrophages through CD46 (measles virus receptor): production of IL-12 p40 and nitric oxide in association with recruitment of protein-tyrosine phosphatase SHP-1 to CD46. J. Immunol., 165, 5143–5152 (2000).
Lu, H. T., Yang, D. D., Wysk, M., Gatti, E., Mellman, I., DAVIS, R. J., and Flavell, R. A., Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J., 18, 1845–1857 (1999).
Ma, X., Chow, J. M., Gri, G., Carra, G., Gerosa, F., Wolf, S. F., Dzialo, R., and Trinchieri, G., The interleukin 12 p40 gene promoter is primed by interferon gamma in monocytic cells. J. Exp. Med., 183, 147–157 (1996).
Ma, X., Neurath, M., Gri, G., and Trinchieri, G., Identification and characterization of a novel Ets-2-related nuclear complex implicated in the activation of the human interleukin-12 p40 gene promoter. J. Biol. Chem., 272, 10389–10395 (1997).
Marengere, L. E., Waterhouse, P., Duncan, G. S., Mittrucker, H. W., Feng, G. S., and Mak, T. W., Regulation of T cell receptor signaling by tyrosine phosphatase SYP association with CTLA-4. Science, 272, 1170–1173 (1996).
Maruyama, S., Sumita, K., Shen, H., Kanoh, M., Xu, X., Sato, M., Matsumoto, M., Shinomiya, H., and Asano, Y., Identification of IFN regulatory factor-1 binding site in IL-12 p40 gene promoter. J. Immunol., 170, 997–1001 (2003).
Murphy, T. L., Cleveland, M. G., Kulesza, P., Magram, J., and Murphy, K. M., Regulation of interleukin 12 p40 expression through an NF-kappa B half-site. Mol. Cell. Biol., 15, 5258–5267 (1995).
Na, S. Y., KangG, B. Y., Chung, S. W., Han, S. J., Ma, X., Trinchieri, G., Im, S. Y., Lee, J. W., and Kim, T. S., Retinoids inhibit interleukin-12 production in macrophages through physical associations of retinoid X receptor and NFkappaB. J. Biol. Chem., 274, 7674–7680 (1999).
Oda, M., Sakitani, K., Kaibori, M., Imoue, T., Kamiyama, Y., and Okumura, T., Vicinal dithiol-binding agent, phenylarsine oxide, inhibits inducible nitric-oxide synthase gene expression at a step of nuclear factor-kappaB DNA binding in hepatocytes. J. Biol. Chem., 275, 4369–4373 (2000).
Plevy, S. E., Gemberling, J. H., Hsu, S., Dorner, A. J., and Smale, S. T., Multiple control elements mediate activation of the murine and human interleukin 12 p40 promoters: evidence of functional synergy between C/EBP and Rel proteins. Mol. Cell. Biol., 17, 4572–4588 (1997).
Ponnappan, U., Trebilcock, G. U., and Zheng, M. Z., Studies into the effect of tyrosine phosphatase inhibitor phenylarsine oxide on NFkappaB activation in T lymphocytes during aging: evidence for altered IkappaB-alpha phosphorylation and degradation. Exp. Gerontol., 34, 95–107 (1999).
Sathish, J. G., Dolton, G., Leroy, F. G., and Matthews, R. J., Loss of Src homology region 2 domain-containing protein tyrosine phosphatase-1 increases CD8+ T cell-APC conjugate formation and is associated with enhanced in vivo CTL function. J. Immunol., 178, 330–337 (2007).
Schmidt, A., Rutledge, S. J., Endo, N., Opas, E. E., Tanaka, H., Wesolowski, G., Leu, C. T., Huang, Z., Ramachandaran, C., Rodan, S. B., and Rodan, G. A., Protein-tyrosine phosphatase activity regulates osteoclast formation and function: inhibition by alendronate. Proc. Natl. Acad. Sci. USA, 93, 3068–3073 (1996).
Shu, U., Kiniwa, M., Wu, C. Y., Maliszewski, C., Vezzio, N., Hakimi, J., Gately, M., and DELESPESSE, G., Activated T cells induce interleukin-12 production by monocytes via CD40-CD40 ligand interaction. Eur. J. Immunol., 25, 1125–1128 (1995).
Sieburth, D., Jabs, E. W., Warrington, J. A., Li, X., Lasota, J., Laforgia, S., Kelleher, K., Huebner, K., Wasmuth, J. J., and Wolf, S. F., Assignment of genes encoding a unique cytokine (IL12) composed of two unrelated subunits to chromosomes 3 and 5. Genomics, 14, 59–62 (1992).
Wachtel, M., Frei, K., Ehler, E., Fontana, A., Winterhalter, K., and Gloor, S. M., Occludin proteolysis and increased permeability in endothelial cells through tyrosine phosphatase inhibition. J. Cell Sci., 112(Pt 23), 4347–4356 (1999).
Wang, I. M., Contursi, C., Masumi, A., Ma, X., Trinchieri, G., and Ozato, K., An IFN-gamma-inducible transcription factor, IFN consensus sequence binding protein (ICSBP), stimulates IL-12 p40 expression in macrophages. J. Immunol., 165, 271–279 (2000).
Wang, J. P., Tsai, J. J., Chen, Y. S., and Hsu, M. F., Stimulation of intracellular Ca2+ elevation in neutrophils by thiol-oxidizing phenylarsine oxide. Biochem. Pharmacol., 69, 1225–1234 (2005).
Wu, X., Zhu, L., Zilbering, A., Mahadev, K., Motoshima, H., Yao, J., and Goldstein, B. J., Hyperglycemia potentiates H(2)O(2) production in adipocytes and enhances insulin signal transduction: potential role for oxidative inhibition of thiol-sensitive protein-tyrosine phosphatases. Antioxid. Redox Signal., 7, 526–537 (2005).
Xia, C. Q. and Kao, K. J., Suppression of interleukin-12 production through endogenously secreted interleukin-10 in activated dendritic cells: involvement of activation of extracellular signal-regulated protein kinase. Scand. J. Immunol., 58, 23–32 (2003).
Yoshimoto, T., Kojima, K., Fukakoshi, T., Endo, Y., Fujita, T., and Nariuchi, H., Molecular cloning and characterization of murine IL-12 genes. J. Immunol., 156, 1082–1088 (1996).
Zhang, S. and Wang, Q., Factors determining the formation and release of bioactive IL-12: regulatory mechanisms for IL-12p70 synthesis and inhibition. Biochem. Biophys. Res. Commun., 372, 509–512 (2008).
Zhu, C., Gagnidze, K., Gemberling, J. H., and Plevy, S. E., Characterization of an activation protein-1-binding site in the murine interleukin-12 p40 promoter. Demonstration of novel functional elements by a reductionist approach. J. Biol. Chem., 276, 18519–18528 (2001).
Zhu, C., Rao, K., Xiong, H., Gagnidze, K., LI, F., Hrovath, C., and Plevy, S., Activation of the murine interleukin-12 p40 promoter by functional interactions between NFAT and ICSBP. J. Biol. Chem., 278, 39372–39382 (2003).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Cho, YC., Lee, K.Y., Kang, B.Y. et al. Enhanced IL-12p40 production by phenylarsine oxide is mediated by cAMP response element in macrophages. Arch. Pharm. Res. 33, 745–751 (2010). https://doi.org/10.1007/s12272-010-0514-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-010-0514-9