Abstract
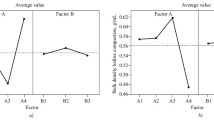
A work has been done to study the individual and interacting effects of formulation variables, using a 23 fractional factorial design. The effects of five variables, namely, relative density of tablets, nature and concentration of binder, compression process, and compression speed on the tensile strength and percent survival of Bacillus subtilis spores in Diclofenac tablet formulations were determined. The effects of these variables were studied both singly and when they interact with each other in two fractional designs (Woolfall, 1964). The first fraction comprised of Nature (N) and Concentration (C) of binder, and Relative density of tablets (D) while in the second fraction, Compression speed (S), Compression process (P) and Relative density of tablets (D) were studied. In the first fraction, concentration of binder had the highest effect on tensile strength with the ranking C > D > N for both DCS (formulation containing Corn starch) and DDCP (formulation containing DCP), and C > N > D for DL (formulation containing Lactose). On the percent survival of Bacillus subtilis, relative density of tablets showed the highest effect with the ranking D > C > N for both DCS and DL, and D > N > C for DDCP. In the second fraction, compression speed generally had a great effect on both tensile strength and percent survival in all the formulations. The results of interactions among the variables showed the highest effect on tensile strength from interaction between concentration of binder and relative density of tablets (C-D) while interaction between compression speed and relative density of tablets (S-D) had the highest effect on percent survival in all the formulations. A fractional factorial design proved suitable in determining the magnitude of both the individual and interacting effects of the variables. The study showed that each of these variables has to be properly considered in producing tablets of satisfactory strength and reduced microbial survival.
Similar content being viewed by others
References
Alander, E. M., Uusi-Penttila, M. S., and Rasmuson, A. C., Characterization of paracetamol agglomerates by image analysis and strength measurement. Powder Technology, 130, 298–306 (2003).
Femi-Oyewo, M. N., Adedokun, M. O., and Olusoga, T. O., Evaluation of the suspending properties of Albizia zygia gum on sulphadimidine suspension. Tropical J. Pharm. Res., 3, 279–284 (2004).
Itiola, O. A. and Pilpel, N., Formulation effects on the mechanical properties of metronidazole tablets. J. Pharm. Pharmacol., 43, 145–147 (1991).
Itiola, O. A. and Pilpel, N., Studies on metronidazole tablet formulations. J. Pharm. Pharmacol., 38, 81–86 (1986a).
Itiola, O. A. and Pilpel, N., Tabletting characteristics of metronidazole formulations. Int. J. Pharm., 31, 99–105 (1986b).
Itiola, O. A. and Pilpel, N., Effects of interacting variables on the disintegration and dissolution of metronidazole tablets. Pharmazie, 51, 987–989 (1996).
Odeku, O. A., Awe, O. O., Popoola, B., Odeniyi, M. A., and Itiola, O. A., Compression and Mechanical Properties of Tablet Formulations Containing Corn, Sweet potato, and Cocoyam starches as Binders. Pharm. Tech., (2005).
Pitt, K. G., Newton, J. M., and Stanley, P., Effects of compaction variables on porosity and material tensile strength of convex-faced aspirin tablets. J. Pharm. Pharmacol., 43, 219–225 (1991).
Plumpton, E. J., Gilbert, P., and Fell, J. T., Effects of spatial distribution of contaminating microorganisms within tablet formulation on subsequent inactivation through compaction. Int. J. Pharm., 30, 237–240 (1986a).
Plumpton, E. J., Gilbert, P., and Fell, J. T., The survival of microorganisms during tabletting. Int. J. Pharm., 30, 241–246 (1986b).
Sujja-areevath, J., Munday, D. L., Cox, P. J., and Khan, K. A., Release characteristics of diclofenac sodium from encapsulated natural gum mini-matrix formulations. Int. J. Pharm., 139, 53–62 (1996).
Woolfall, R. C., An approach to product formulation. Soap Perfum. Cosmet., 37, 965–970 (1964).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ayorinde, J.O., Itiola, O.A. Individual and interacting effects of formulation variables on the tensile strength and microbial survival in diclofenac tablets. Arch. Pharm. Res. 33, 395–403 (2010). https://doi.org/10.1007/s12272-010-0308-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-010-0308-0