Abstract
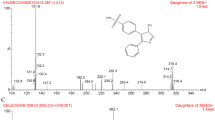
PAR-5359, 3-(4-{2-[4-(4-Chloro-phenyl)-3,6-dihydro-2H-pyridin-1-yl]-ethoxy}-phenyl)-2-ethoxypropionic acid, is a well-balanced PPARα/γ dual agonist with the excellent antihyperglycemic and hypolipidemic activities. A reliable, selective and sensitive high-performance liquid chromatography with electrospray ionization tandem mass spectrometry was developed for the determination of PAR-5359 in rat plasma. PAR-5359 was twice extracted from rat plasma using methyl tert-butyl ether at neutral pH. The analytes were separated on an Allure Biphenyl column with the mobile phase of 78% methanol in 10 mM ammonium formate (pH 3.0) and detected by tandem mass spectrometry in the selective reaction monitoring mode. The calibration curve was linear (r = 0.9993) over the concentration range of 2.00–1000.0 ng/mL and the lower limit of quantification was 2.00 ng/mL using 50 μL plasma sample. The coefficient of variation and relative error at four QC levels were 1.2 to 12.3% and −2.5 to 6.3%, respectively. The present method was successfully applied to the pharmacokinetic study of PAR-5359 after oral dose of PAR-5359 at a dose of 1 mg/kg to male SD rats.
Similar content being viewed by others
References
Balakumar, P., Rose, M., Ganti, S. S., Krishan, P., and Singh, M., PPAR dual agonists: are they opening Pandora’s Box? Pharmacol. Res., 56, 91–98 (2007).
Chaput, E., Saladin, R., Silvestre, M., and Edgar, A. D., Fenofibrate and rosiglitazone lower serum triglycerides with opposing effects on body weight. Biochem. Biophys. Res. Commun., 271, 445–450 (2000).
Han, H. O., Kim, S. H., Kim, K. H., Hur, G. C., Yim, H. J., Chung, H. K., Woo, S. H., Koo, K. D., Lee, C. S., Koh, J. S., and Kim, G. T., Design and synthesis of oxime ethers of alpha-acyl-beta-phenylpropanoic acids as PPAR dual agonists. Bioorg. Med. Chem. Lett., 17, 937–941 (2007).
Hsieh, Y., HPLC-MS/MS in drug metabolism and pharmacokinetic screening. Expert Opin. Drug Metab. Toxicol., 4, 93–101 (2008).
Ji, H. Y., Jeong, D. W., Kim, Y. H., Kim, H. H., Yoon, Y. S., Lee, K. C., and Lee, H. S., Determination of gabapentin in human plasma using hydrophilic interaction liquid chromatography with tandem mass spectrometry. Rapid Commun. Mass Spectrom., 20, 2127–2132 (2006).
Kim, E., Park, C. S., Han, T., Bae, M. H., Chong, W., Lee, C. H., Shin, Y. A., Ahn, B. N., Kim, M. K., Shin, C. Y., Son, M. H., Kim, J. K., Moon, H. S., Shim, H. J., Kim, E. J., Kim, S. H., Lim, J. I., and Lee, C. H., Design, synthesis, and evaluation of novel aryl-tetrahydropyridine PPARα/γ dual agonists. Bioorg. Med. Chem. Lett., 18, 4993–4996 (2008a).
Kim, M. K., Chae, Y. N., Son, M. H., Kim, S. H., Kim, J. K., Moon, H. S., Park, C. S., Bae, M. H., Kim, E., Han, T., Choi, H., Shin, Y. A., Ahn, B. N., Lee, C. H., Lim, J. I., and Shin, C. Y., PAR-5359, a well-balanced PPARα/γ dual agonist, exhibits equivalent antidiabetic and hypolipidemic activities in vitro and in vivo. Eur. J. Pharmacol., 595, 119–125 (2008b).
Korfmacher, W. A., Principles and applications of LC-MS in new drug discovery. Drug Discov. Today, 10, 1357–1367 (2005).
Lee, H. W., Ji, H. Y., Park, E. S., Lee, K. C., and Lee, H. S., Hydrophilic interaction chromatographytandem mass spectrometric analysis of irbesartan in human plasma: Application to pharmacokinetic study of irbesartan. J. Sep. Sci., 32, 2353–2358 (2009).
Matuszewski, B. K., Constanzer, M. L., and Chavez-Eng, C. M., Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal. Chem., 75, 3019–3030 (2003).
Paek, B., Ji, H. Y., Kim, M. S., Lee, G., and Lee, H. S., Metabolism of a new P-glycoprotein inhibitor HM-30181 in rats using liquid chromatography/electrospray mass spectrometry. Rapid Commun. Mass Spectrom., 20, 1457–1462 (2006).
Watts, G. F. and Dimmitt, S. B., Fibrates, dysliporoteinaemia and cardiovascular disease. Curr. Opin. Lipidol., 10, 561–574 (1999).
White, R. E., High-throughput screening in drug metabolism and pharmacokinetic support of drug discovery. Annu. Rev. Pharmacol. Toxicol., 40, 133–157 (2000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, D.K., Park, E.J., Jeong, J.H. et al. Liquid chromatography-tandem mass spectrometry of a new PPARα/γ dual agonist PAR-5359 in rat plasma. Arch. Pharm. Res. 32, 1743–1748 (2009). https://doi.org/10.1007/s12272-009-2212-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-009-2212-z