Abstract
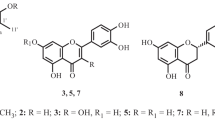
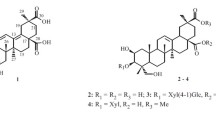
A new triterpenoid, 30-hydroxyalphitolic acid 1, and eight known triterpenoids, alphitolic acid 2, lupenol 3, 3-acetoxy-olean-18-en-28-oic acid 4, betulinic acid 5, ursolic acid 6, betulinic acid 3-O-caffeate 7, morolic acid 3-O-caffeate 8, and ursolic acid 3-O-caffeate 9, were isolated from Callistemon lanceolatus. Their structures were determined using spectroscopic techniques, which included 1D- and 2D-NMR. All compounds were evaluated for the inhibition of LPS-induced nitric oxide production in murine macrophage RAW264.7 cells. Betulinic acid 3-O-caffeate 7 showed a moderate inhibitory effect on nitric oxide production with IC50 value of 15.4 μM.
Similar content being viewed by others
References
Burns, D., Reynolds, W. F., Buchanan, G., Reese, P. B., and Enriquez, R. G., Assignment of 1H and 13C spectra and investigation of hindered side-chain rotation in lupeol derivatives. Magn. Reson. Chem., 38, 488–493 (2000).
Cichewicz, R. H. and Kouzi, S. A., Chemistry, biological activity, and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Med. Res. Rev., 24, 90–114 (2004).
Hamburger, M., Riese, U., Graf, H., Melzig, M. F., Ciesielski, S., Baumann, D., Dittmann, K., and Wegner, C., Constituents in evening primrose oil with radical scavenging, cyclooxygenase, and neutrophil elastase inhibitory activities. J. Agric. Food Chem., 50, 5533–5538 (2002).
Huq, F. and Misra, L. N., An alkenol and C-methylated flavones from Callistemon lanceolatus leaves. Planta Med., 63, 369–370 (1997).
Hwang, B. Y., Lee, J. H., Koo, T. H., Kim, H. S., Hong, Y. S., Ro, J. S., Lee, K. S., and Lee, J. J., Kaurane diterpenes from Isodon japonicus inhibit nitric oxide and prostaglandin E2 production and NF-κB activation in LPS-stimulated macrophage RAW264.7 cells. Planta Med., 67, 406–410 (2001).
Jun, C. D., Choi B. M., Hoon, R., Um, J. Y., Kwak, H. J., and Lee, B. S., Synergistic cooperation between phorbol ester and IFN-gamma for induction of nitric oxide synthesis in murine peritoneal macrophages. J. Immunol., 153, 3684–3690 (1994).
Kuang, H. S., Kasai, R., Ohtani, K., Liu, Z. S., Yuan, C. S., and Tanaka O., Chemical constituents of pericarps of Rosa davurica Pall., a traditional Chinese medicine. Chem. Pharm. Bull., 37, 2232–2233 (1989).
Li, N., Yu, F., and Yu, S. S., Triterpenoids from Erythrophleum fordii. Acta Bot. Sin., 46, 371–374 (2004).
Lounasmaa, M., Puri, H. S., Widen, C. J., Phloroglucinol derivatives of Callistemon lanceolatus leaves. Phytochemistry, 16, 1851–1852 (1977).
Mahmoud, I. I., Moharram, F. A., Marzouk, M. S. A., Linscheid, M. W., and Saleh, M. I., Polyphenolic constituents of Callistemon lanceolatus leaves. Pharmazie, 57, 494–496 (2002).
Marzouk, M. S. A., An acylated flavonol glycoside and hydrolysable tannins from Callistemon lanceolatus flowers and leaves. Phytochem. Anal., 19, 541–549 (2008).
Mimaki, Y., Kuroda, M., Yokosuka, A., Harada, H., Fukushima, M., and Sashida, Y., Triterpenes and saponins from the stems of Akebia trifoliate. Chem. Pharm. Bull., 51, 960–965 (2003).
Nathan, C., Nitric oxide as a secretory product of mammalian cells. FASEB J., 6, 3051–3064 (1992).
Pan, H., Lundgren, L. N., and Andersson, R., Triterpene caffeates from bark of Betula pubescens. Phytochemistry, 37, 795–799 (1994).
Pandey, D. K., Chandra, H., and Tripathi, N. N., Volatile fungitoxic activity of some higher plants with special reference to that of Callistemon lanceolatus DC. J. Phytopathol., 105, 175–182 (1982).
Seebacher, W., Simic, N., Weis, R., Safand, R., and Kunert, O., Complete assignment of 1H and 13C NMR resonance of oleanolic acid, 18α-oleanolic acid, ursolic acid and their 11-oxo derivatives. Magn. Reson. Chem., 41, 636–638 (2003).
Simpson, M. G., Plant Systematics. Burlington, MA, Elsevier Academic press, Boston, pp. 256–259, (2006).
Sudhakar, M. and Raju, D. B., Chemical composition and antimicrobial activities of essential oil of Indian Callistemon lanceolatus leaves. Int. J. Chem. Sci., 3, 513–516 (2005).
Tanachatchairatana, T., Bremner, J. B., Chokchaisiri, R., and S, A., Antimycobacterial activity of cinnamate-based esters of the triterpenes betulinic, oleanolic and ursolic acids. Chem. Pharm. Bull., 56, 194–198 (2008).
Varma, R. S. and Parthasarathy, M. R., Triterpenoids of Callistemon lanceolatus leaves. Phytochemistry, 14, 1675–1676 (1975).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jeong, W., Hong, S.S., Kim, N. et al. Bioactive triterpenoids from Callistemon lanceolatus . Arch. Pharm. Res. 32, 845–849 (2009). https://doi.org/10.1007/s12272-009-1605-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-009-1605-3