Abstract
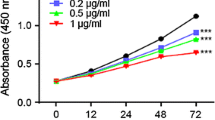
Ginsenoside Rg3, the main constituent isolated from Panax ginseng, has been of interest for use as a cancer preventive or therapeutic agent. We investigated here whether Rg3 can inhibit the activity of NF-κB, a key transcriptional factor constitutively activated in colon cancer that confers cancer cell resistance to chemotherapeutic agents. To investigate whether RG3 can suppress activation of NF-κB, and thus inhibit cancer cell growth, we examined the susceptibility of colon cancer cells (SW620 and HCT116) to treatment with Rg3 (25, 50, 75, 100 μM) and RG3-induced activation of NF-κB. RG3 dose-dependently inhibited cancer cell growth through induction of apoptosis and decreased NF-κB activity. In a further study of compounds in colon cancer, we used half of the IC50 dose, values in combined treatments of Rg3 (50 μM) with conventional agents — docetaxel (5 nM), paclitaxel (10 nM) cisplatin (10 μM) and doxorubicin (2 μM). Compared to treatment with Rg3 or chemotherapy alone, combined treatment was more effective (i.e., there were synergistic effects) in the inhibition of cancer cell growth and induction of apoptosis and these effects were accompanied by significant inhibition of NF-κB activity. NF-κB target gene expression of apoptotic cell death proteins (Bax, caspase-3, caspase-9) was significantly enhanced, but the expression of anti-apoptotic genes and cell proliferation marker genes (Bcl-2, inhibitor of apoptosis protein (IAP-1) and X chromosome IAP (XIAP), Cox-2, c-Fos, c-Jun and cyclin D1) was significantly inhibited by the combined treatment compared to Rg3 or docetaxel alone. These results indicate that ginsenoside Rg3 inhibits NF-κB, and enhances the susceptibility of colon cancer cells to docetaxel and other chemotherapeutics. Thus, ginsenoside Rg3 could be useful as an anti-cancer or adjuvant anti-cancer agent.
Similar content being viewed by others
References
Amer, A. B. and Baltimore, D., An essential role for NF-κB in preventing TNF-α induced cell death. Science, 274, 782–784 (1996).
Ban, J. O., Yuk, D. Y., Woo, K. S., Kim, T. M., Lee, U. S., Jeong, H. S., Kim, D. J., Chung, Y. B., Hwang, B. Y., Oh, K. W., and Hong, J. T., Inhibition of cell growth and induction of apoptosis via inactivation of NF-kappaB by a sulfurcompound isolated from garlic in human colon cancer cells. J. Pharmacol. Sci., 104, 374–383 (2007).
Barkett, M. and Gilmore, T. D., Control of apoptosis by Rel/NF-κB transcription factors. Oncogene, 18, 6910–6924 (1999)
Bednarski, B. K., Ding, X., Coombe, K., Baldwin, A. S, Kim, H. J., Active roles for inhibitory kappaB kinases alpha and beta in nuclear factor-kappaB-mediated chemoresistance to doxorubicin. Mol. Cancer. Ther., 7, 1827–1835 (2008).
Bours, V., Dejardin, E., Goujon-Letawe, F., Merville, M. P., and Castronovo, V., The NF-kappa B transcription factor and cancer: high expression of NF-kappa B- and I kappa B related proteins in tumor cell lines. Biochem. Pharmacol., 47, 145–149 (1994).
Chuang, S. E., Yeh, P. Y., and Lu, Y. S., Basal levels and patterns of anticancer drug-induced activation of nuclear factor-kappaB (NF-kappaB), and its attenuation by tamoxifen, dexamethasone, and curcumin in carcinoma cells. Biochem. Pharmacol., 63, 1709–1716 (2002).
Cortes, J. E. and Pazdur, R., Docetaxel. J. Clin. Oncol., 13, 2643–2655 (1995).
Cusack, J. C. Jr., Liu, R., and Baldwin, A. S. Jr., Inducible chemoresistance to 7-ethyl-10-[4-(1-piperidino)-1-piperidino]-carbonyloxycamptothe cin (CPT-11) in colorectal cancer cells and a xenograft model is overcome by inhibition of nuclear factor-kappaB activation. Cancer Res., 60, 2323–2330 (2000).
Diaz-Rubio, E., New chemotherapeutic advances in pancreatic, colorectal, and gastric cancers. Oncologist., 9, 282–294 (2004)
Dong, Q. G., Sclabas, G. M., and Fujioka, S., The function of multiple IkappaB: NF-kappaB complexes in the resistance of cancer cells to Taxol-induced apoptosis. Oncogene, 21, 6510–6519 (2002).
Ferraresi, V., Milella, M., Vaccaro, A., D’Ottavio, A. M., Papaldo, P., Nisticò, C., Thorel, M. F., Marsella, A., Carpino, A., Giannarelli, D., Terzoli, E., and Cognetti, F., Toxicity and activity of docetaxel in anthracycline-pretreated breast cancer patients: a phase II study. Am. J. Clin. Oncol., 23, 132–139 (2000).
Fossella, F. V., DeVore, R., Kerr, R. N., Crawford, J., Natale, R. R., Dunphy, F., Kalman, L., Miller, V., Lee, J. S., Moore, M., Gandara, D., Karp, D., Vokes, E., Kris, M., Kim, Y., Gamza, F., and Hammershaimb, L., Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J. Clin. Oncol., 18, 2354–2362 (2000).
Garg, A. and Aggarwal, B. B., Nuclear transcription factor-kappaB as a target for cancer drug development. Leukemia, 16, 1053–1068 (2002).
Greten, F. R., Eckmann, L., Greten, T. F., Park, J. M., Li, Z. W., and Egan, L. J., IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell, 118, 285–296 (2004).
Helms, S., Cancer prevention and therapeutics: Panax ginseng. Altern. Med. Rev., 9, 259–274 (2004).
Huang, Y., Johnson, K. R., Norris, J. S., and Fan, W., Nuclear factor-kappaB/ IkappaB signaling pathway may contribute to the mediation of paclitaxel-induced apoptosis in solid tumor cells. Cancer Res., 60, 4426–4432 (2000).
Kasibhatla, S., Brunner, T., Genestier, L., Echeverri, F., Mahboubi, A., and Green, D. R., DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-κB and AP-1. Mol. Cell., 1, 543–551 (1998).
Keum, Y. S., Han, S. S., Chun, K. S., Park, K. K., Park, J. H., Lee, S. K., and Surh, Y. J., Inhibitory effects of the ginsenoside Rg3 on phorbol ester-induced cyclooxygenase-2 expression, NF-kappaB activation and tumor promotion. Mutat. Res., 524, 75–85 (2003).
Lee, S. H., Lee, C. W., Lee, J. W., Choi, M. S., Son, D. J., Chung, Y. B., Lee, C. K., Oh, K. W., Moon, D. C., Kwon, B. M., and Hong, J. T., Induction of apoptotic cell death by 2′-hydroxycinnamaldehyde is involved with ERK-dependent inactivation of NF-kappaB in TNF-alpha-treated SW620 colon cancer cells. Biochem. Pharmacol., 70, 1147–1157 (2005).
Li, Y., Ellis, K. L., and Ali, S., Apoptosis-inducing effect of chemotherapeutic agents is potentiated by soy isoflavone genistein, a natural inhibitor of NF-kappaB in BxPC-3 pancreatic cancer cell line. Pancreas., 28, 90–95 (2004).
Lin, Z. P., Boller, Y. C., Amer, S. M., Russell, R. L., Pacelli, K. A., Patierno, S. R., and Kennedy, K. A., Prevention of brefeldin A-induced resistance to teniposide by the proteasome inhibitor MG-132: involvement of NF-κB activation in drug resistance. Cancer Res., 58, 3059–3065 (1998).
Lind, D. S., Hochwald, S. N., Malaty, J., Rekkas, S., Hebig, P., Mishra, G., Moldawer, L. L., Copeland, E. M. 3rd., and MacKay, S., Nuclear factor-kappa B is upregulated in colorectal cancer. Surgery, 130, 363–369 (2001).
Mabuchi, S., Ohmichi, M., Nishio, Y., Hayasaka, T., Kimura, A., Ohta, T., Kawagoe, J., Takahashi, K., Yada-Hashimoto, N., Seino-Noda, H., Sakata, M., Motoyama, T., Kurachi, H., Testa, J. R., Tasaka, K., and Murata, Y., Inhibition of inhibitor of nuclear factor-kappaB phosphorylation increases the efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Clin. Cancer Res., 10, 7645–7654 (2004).
Marianneau, P., Cardona, A., Edelman, L., Deubel, V., and Despres, P., Dengue virus replication in human hepatoma cells activates NF-κB which in turn induces apoptotic cell death. J. Virol., 71, 3244–3249 (1997).
Millikan, R., Baez, L., Banerjee, T., Wade, J., Edwards, K., Winn, R., Smith, T. L., and Logothetis, C., Randomized phase 2 trial of ketoconazole and ketoconazole/doxorubicin in androgen independent prostate cancer. Urol. Oncol., 6, 111–115 (2001).
Okada, S., Sakata, Y., Matsuno, S., Kurihara, M., Sasaki, Y., Ohashi, Y., and Taguchi, T., Phase II study of docetaxel in patients with metastatic pancreatic cancer: a Japanese cooperative study. Cooperative Group of Docetaxel for Pancreatic Cancer in Japan. Br. J. Cancer, 80, 438–443 (1999).
Oyaizu, H., Adachi, Y., and Okumura, T., Proteasome inhibitor 1 enhances paclitaxel-induced apoptosis in human lung adenocarcinoma cell line. Oncol. Rep., 8, 825–829 (2001).
Perkins, N. D., The Rel/NF-κB family: friend and foe. Trends. Biochem. Sci., 25, 434–440 (2000).
Pikarsky, E., Porat, R. M., Stein, I., Abramovitch, R., Amit, S., Kasem, S., NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature, 431, 461–466 (2004).
Qin, Z. H., Wang, Y., Nakai, M., and Chase, T. N., Nuclear factor-κB contributes to excitotoxin-induced apoptosis in rat striatum. Mol. Pharmacol., 53, 33–42 (1998).
Ryan, D. P., Kulke, M. H., Fuchs, C. S., Grossbard, M. L., Grossman, S. R., Morgan, J. A., Earle, C. C., Shivdasani, R., Kim, H., Mayer, R. J., and Clark, J. W., A Phase II study of gemcitabine and docetaxel in patients with metastatic pancreatic carcinoma. Cancer, 94, 97–103 (2002).
Schneider, A., Martin-Villalba, A., Weih, F., Vogel, J., Wirth, T., and Schwaninger, M., NF-κB is activated and promotes cell death in focal cerebral ischemia. Nat. Med., 5, 554–559 (1999).
Singh, S. and Bhat, M. K., Carboplatin induces apoptotic cell death through downregulation of constitutively active nuclear factor-kappaB in human HPV-18 E6-positive HEp-2 cells. Biochem. Biophys. Res. Commun., 318, 346–353 (2004).
Son, D. J., Park, M. H., Chae, S. J., Moon, S. O., Lee, J. W., Song, H. S., Moon, D. C., Kang, S. S., Kwon, Y. E., and Hong, J. T. Inhibitory effect of snake venom toxin from Vipera lebetina turanica on hormone-refractory human prostate cancer cell growth: induction of apoptosis through inactivation of nuclear factor kappaB. Mol. Cancer Ther., 6, 675–683 (2007).
Strasser, F., Hailemariam, S., Hauser, M., and Pestalozzi, B. C., A 53-year-old patient with unilateral pulmonary infiltrates following a single dose of mitomycin, vindesine and cisplatin chemotherapy for non-small-cell lung cancer. Respiration, 66, 562–572 (1999).
Suh, Y., Afaq, F., Johnson, J. J., and Mukhtar, H., A plant flavonoid fisetin induces apoptosis in colon cancer cells by inhibition of COX2 and Wnt/EGFR/NF-{kappa}B signaling pathways. Carcinogenesis, Epub ahead of print, (2008)
Tedesco, K. L., Thor, A. D., Johnson, D. H., Shyr, Y., Blum, K. A., Goldstein, L. J., Gradishar, W. J., Nicholson, B. P., Merkel, D. E., Murrey, D., Edgerton, S., and Sledge, G. W. Jr., Docetaxel combined with trastuzumab is an active regimen in HER-2 3+ overexpressing and fluorescent in situ hybridization-positive metastatic breast cancer: a multi-institutional phase II trial. J. Clin. Oncol., 22, 1071–1077 (2004).
Tyagi, A. K., Singh, R. P., Agarwal, C., Chan, D. C., and Agarwal, R., Silibinin strongly synergizes human prostate carcinoma DU145 cells to doxorubicin-induced growth Inhibition, G2-M arrest, and apoptosis. Clin. Cancer Res., 8, 3512–9 (2002).
Van, Antwerp. D. J., Martin, S. J., Kafri, T., Green, D. R., and Verma, I. M., Suppression of TNF-α-induced apoptosis by NF-κB. Science., 274, 787–789 (1996).
Waddick, K. G., and Uckun, F. M., Innovative treatment programs against cancer: II. Nuclear factor-κB (NF-κB) as a molecular target. Biochem. Pharmacol., 57, 9–17 (1999).
Wang, C. Y., Cusack, J. C., Liu, R., and Baldwin, A. S. Jr., Control of inducible chemoresistance: enhanced antitumor therapy through increased apoptosis by inhibition of NF-κB. Nat. Med., 5, 412–417 (1999).
Wang, C. Y., Mayo, M. W., and Baldwin, A. S. Jr., TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science., 274, 784–787 (1996).
Weldon, C. B., Burow, M. E., Rolfe, K. W., Clayton, J. L., Jaffe, B. M., and Beckman B. S., NF-kappa B-mediated chemoresistance in breast cancer cells. Surgery,130, 143–150 (2001).
Xiuli, W., Yan, W., Wenqian, Z., and Yixuan, Z., Fungal biotransformation of ginsenoside Rg3. Wei Sheng Wu Xue Bao, 48, 1181–5 (2008).
Yun, T. K., Experimental and epidemiological evidence on non-organ specific cancer preventive effect of Korean ginseng and identification of active compounds. Mutat. Res., 523–524, 63–74 (2003).
Zhang, H., Morisaki, T., and Nakahara, C., PSK-mediated NF-kappaB inhibition augments docetaxel-induced apoptosis in human pancreatic cancer cells NOR-P1. Oncogene, 22, 2088–2096 (2003).
Zhao, Y., Wang, W., Han, L., Rayburn, E. R., Hill, D. L., Wang, H., and Zhang, R., Isolation, structural determination, and evaluation of the biological activity of 20(S)-25-methoxyl-dammarane-3beta, 12beta, 20-triol [20(S)-25-OCH3-PPD], a novel natural product from Panax notoginseng. Med. Chem., 3, 51–60 (2007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, S.M., Lee, S.Y., Yuk, D.Y. et al. Inhibition of NF-κB by ginsenoside Rg3 enhances the susceptibility of colon cancer cells to docetaxel. Arch. Pharm. Res. 32, 755–765 (2009). https://doi.org/10.1007/s12272-009-1515-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-009-1515-4