Abstract
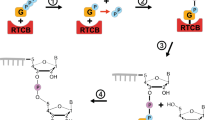
Proteins that fail to fold correctly in the endoplasmic reticulum (ER) are retrotranslocated back into the cytosol where they become ubiquitinated and are eventually degraded by the proteasome. This process is called ER-associated protein degradation (ERAD). Recent research revealed that the ER-membrane protein Hrd1, an E3 ubiquitin ligase, forms a minimal system to translocate misfolded proteins across the membrane of the ER.
Similar content being viewed by others
Literatur
Lippincott-Schwartz J, Bonifacino JS, Yuan LC et al. (1988) Degradation from the endoplasmic reticulum: disposing of newly synthesized proteins. Cell 54:209–220
Sommer T, Jentsch S (1993) A protein translocation defect linked to ubiquitin conjugation at the endoplasmic reticulum. Nature 365:176–179
Knop M, Finger A, Braun T et al. (1996) Der1, a novel protein specifically required for endoplasmic reticulum degradation in yeast. EMBO J 15:753–763
Christianson JC, Ye Y (2014) Cleaning up in the endoplasmic reticulum: ubiquitin in charge. Nat Struct Mol Biol 21:325–335
Foresti O, Rodriguez-Vaello V, Funaya C et al. (2014) Quality control of inner nuclear membrane proteins by the Asi complex. Science 346:751–755
Khmelinskii A, Blaszczak E, Pantazopoulou M et al. (2014) Protein quality control at the inner nuclear membrane. Nature 516:410–413
Mehnert M, Sommer T, Jarosch E (2014) Der1 promotes movement of misfolded proteins through the endoplasmic reticulum membrane. Nat Cell Biol 16:77–86
Carvalho P, Stanley AM, Rapoport TA (2010) Retrotranslocation of a misfolded luminal ER protein by the ubiquitin-ligase Hrd1p. Cell 143:579–591
Stein A, Ruggiano A, Carvalho P et al. (2014) Key steps in ERAD of luminal ER proteins reconstituted with purified components. Cell 158:1375–1388
Baldrige RD, Rapoport TA (2016) Autoubiquitination of the Hrd1 ligase triggers protein retrotranslocation in ERAD. Cell 166:394–407
Author information
Authors and Affiliations
Corresponding author
Additional information
Alexander Stein 1998–2003 Biochemiestudium an der FU Berlin. 2003–2008 Promotion am Max-Planck-Institut für biophysikalische Chemie, Göttingen, dort 2008–2009 Postdoc. 2010–2014 Postdoc bei Prof. Dr. T. A. Rapoport, Harvard Medical School, Boston, MA, USA. Seit 2014 Gruppenleiter am Max-Planck-Institut für biophysikalische Chemie, Göttingen.
Rights and permissions
About this article
Cite this article
Stein, A. Retrotranslokation über die ER-Membran durch die Ubiquitinligase Hrd1. Biospektrum 22, 459–461 (2016). https://doi.org/10.1007/s12268-016-0711-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12268-016-0711-2