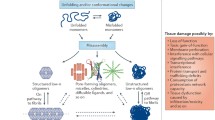
Abstract
Aberrant protein aggregation into amyloid fibrils is linked to cell degeneration and the pathogenesis of a number of fatal diseases including Alzheimer’s disease and type 2 diabetes. Here biomolecular recognition principles and peptide-based strategies towards inhibition of protein aggregation and associated cell damage in these two yet incurable amyloid diseases are reviewed.
Similar content being viewed by others
Literatur
Westermark P (2005) Aspects on human amyloid forms and their fibril polypeptides. FEBS J 272:5942–5949
Chiti F, Dobson CM (2006) Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem 75:333–366
Soto C (2003) Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci 4:49–60
Tjernberg LO, Näslund J, Lindquist F et al. (1996) Arrest of b-amyloid fibril formation by a pentapeptide ligand. J Biol Chem 271:8545–8548
Tenidis K, Waldner M, Bernhagen J et al. (2000) Identification of a penta- and hexapeptide of islet amyloid polypeptide (IAPP) with amyloidogenic and cytotoxic properties. J Mol Biol 295:1055–1071
Sciarretta KL, Gordon DJ, Meredith SC (2006) Peptidebased inhibitors of amyloid assembly. Methods Enzymol 413:273–312
Kapurniotu A, Schmauder A, Tenidis K (2002) Structurebased design and study of non-amyloidogenic, double Nmethylated IAPP amyloid core sequences as inhibitors of IAPP amyloid formation and cytotoxicity. J Mol Biol 315:339–350
Tatarek-Nossol M, Yan LM, Schmauder A et al. (2005) Inhibition of IAPP amyloid fibril formation and apoptotic cell death by a designed IAPP amyloid core-containing hexapeptide. Chem Biol 12:797–809
Kapurniotu A, Bernhagen J, Brunner H (1997) Peptide als Agonisten und/oder Inhibitoren der Amyloidbildung und Zytotoxizität sowie ihre Verwendung bei der Alzheimerschen Krankheit, beim Typ II Diabetes mellitus und bei spongiformen Encephalopathien. DE-A1 197 25 619
Westermark P, Andersson A, Westermark GT (2011) Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev 91:795–826
Schmitz O, Brock B, Rungby J (2004) Amylin agonists: a novel approach in the treatment of diabetes. Diabetes 53:233–238
Yan LM, Tatarek-Nossol M, Velkova A et al. (2006) Design of a mimic of nonamyloidogenic and bioactive human islet amyloid polypeptide (IAPP) as nanomolar affinity inhibitor of IAPP cytotoxic fibrillogenesis. Proc Natl Acad Sci USA 103:2046–2051
Li L, Hölscher C (2007) Common pathological processes in Alzheimer disease and type 2 diabetes: a review. Brain Res Rev 56:384–402
O’Nuallain B, Williams AD, Westermark P et al. (2004) Seeding specificity in amyloid growth induced by heterologous fibrils. J Biol Chem 279:17490–17499
Yan LM, Velkova A, Tatarek-Nossol M et al. (2007) IAPP mimic blocks Ab cytotoxic self-assembly: cross-suppression of amyloid toxicity of Ab and IAPP suggests a molecular link between Alzheimer’s disease and type II diabetes. Angew Chem Int Ed Engl 46:1246–1252
Andreetto E, Yau LM, Tatarek-Nossol M et al. (2010) Identification of hot regions of the Abeta-IAPP interaction interface as high-affinity binding sites in both cross- and self-association. Angew Chem Ed Engl 49:3081–3085
Author information
Authors and Affiliations
Corresponding author
Additional information
Aphrodite Kapurniotu Jahrgang 1961. 1979–1985 Chemiestudium an den Universitäten Athen und Tübingen. 1990 Promotion an der Universität Tübingen. 1990–1992 wissenschaftliche Angestellte an der Universität Tübingen. 1992–1994 Postdoktorandin an der Rutgers University, NJ, USA. 1994–1995 Senior Scientist am Picower Institute for Medical Research, NY, USA. 1994–2007 Gruppenleiterin an der Universität Tübingen und der RWTH Aachen. 2001 Habilitation im Fach Biochemie an der Universität Tübingen. 2006 Ernennung zur apl. Professorin RWTH Aachen. Seit 2007 Professorin für Peptidbiochemie an der TU München.
Rights and permissions
About this article
Cite this article
Kapurniotu, A. Strategien zur Inhibition der Protein-aggregation bei Amyloidkrankheiten. Biospektrum 18, 734–736 (2012). https://doi.org/10.1007/s12268-012-0255-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12268-012-0255-z