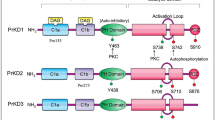
Abstract
Targeted therapy, such as tyrosine kinase inhibitors (TKIs), has been approved to manage various cancer types. However, TKI-induced cardiotoxicity is a limiting factor for their use. This issue has raised the need for investigating potential cardioprotective techniques to be combined with TKIs. Ribosomal S6-kinases (RSKs) are a downstream effector of the mitogen-activated-protein-kinase (MAPK) pathway; specific RSK isoforms, such as RSK1 and RSK2, have been expressed in cancer cells, in which they increase tumour proliferation. Selective targeting of those isoforms would result in tumour suppression. Moreover, activation of RSKs expressed in the heart has resulted in cardiac hypertrophy and arrhythmia; thus, inhibiting RSKs would result in cardio-protection. This review article presents an overview of the usefulness of RSK inhibitors that can be novel agents to be assessed in future research for their effect in reducing cancer proliferation, as well as protecting the heart from cardiotoxicity induced by TKIs.
Graphical Abstract






Similar content being viewed by others
References
Ahmad FB, Anderson RN. The leading causes of death in the US for 2020. JAMA. 2021;325(18):1829–30.
Curigliano G, et al. Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA Cancer J Clin. 2016;66(4):309–25.
Teske A, et al. Cardio-oncology: an overview on outpatient management and future developments. Neth Hear J. 2018;26(11):521–32.
Wickramasinghe CD, et al. Concepts in cardio-oncology: definitions, mechanisms, diagnosis and treatment strategies of cancer therapy-induced cardiotoxicity. Future Oncol. 2016;12(6):855–70.
Han X, Zhou Y, Liu W. Precision cardio-oncology: understanding the cardiotoxicity of cancer therapy. NPJ Precis Oncol. 2017;1(1):1–11.
Zheng PP, Li J, Kros JM. Breakthroughs in modern cancer therapy and elusive cardiotoxicity: critical research-practice gaps, challenges, and insights. Med Res Rev. 2018;38(1):325–76.
Sheng CC, et al. 21st century cardio-oncology: identifying cardiac safety signals in the era of personalized medicine. JACC: Basic Transl Sci. 2016;1(5):386–98.
Casalvieri KA, et al. Selective targeting of RSK isoforms in cancer. Trends Cancer. 2017;3(4):302–12.
Paul MK, Mukhopadhyay AK. Tyrosine kinase–role and significance in cancer. Int J Med Sci. 2004;1(2):101.
Gorini S, De Angelis A, Berrino L, Malara N, Rosano G, Ferraro E. Chemotherapeutic drugs and mitochondrial dysfunction: focus on doxorubicin, trastuzumab, and sunitinib. Oxidat Med Cell Longev. 2018;2018:15. https://doi.org/10.1155/2018/7582730
Chen MH, Kerkelä R, Force T. Mechanisms of cardiac dysfunction associated with tyrosine kinase inhibitor cancer therapeutics. Circ Res. 2008;118(1):84–95.
Greineder CF, Kohnstamm S, Ky B. Heart failure associated with sunitinib: lessons learned from animal models. Curr Hypertens Rep. 2011;13(6):436–41.
Chaar M, Kamta J, Ait-Oudhia S. Mechanisms, monitoring, and management of tyrosine kinase inhibitors-associated cardiovascular toxicities. Onco Targets Ther. 2018;11:6227–37.
Mulas O, et al. Arterial hypertension and tyrosine kinase inhibitors in chronic myeloid leukemia: a systematic review and meta-analysis. Front Pharmacol. 2021;12:674748.
Shyam Sunder S, Sharma UC, Pokharel S. Adverse effects of tyrosine kinase inhibitors in cancer therapy: pathophysiology, mechanisms and clinical management. Signal Transduct Target Ther. 2023;8(1):262.
Mercurio V, et al. Pulmonary hypertension induced by anticancer drugs. In: Russo A, et al., editors. Cardiovascular complications in cancer therapy. Cham: Springer International Publishing; 2019. p. 133–9.
McMullen CJ, et al. Sunitinib and imatinib display differential cardiotoxicity in adult rat cardiac fibroblasts that involves a role for calcium/calmodulin dependent protein kinase II. Front Cardiovasc Med. 2020;7:630480.
Singh AP, et al. Ponatinib-induced cardiotoxicity: delineating the signalling mechanisms and potential rescue strategies. Cardiovasc Res. 2019;115(5):966–77.
Vallakati A, et al. Management of cancer therapeutics–related cardiac dysfunction. J Heart Fail Clin. 2018;14(4):553–67.
Bloom MW, et al. Cancer therapy–related cardiac dysfunction and heart failure: part 1: definitions, pathophysiology, risk factors, and imaging. J Circ: Heart Fail. 2016;9(1):e002661.
Koutsoukis A, et al. Cardio-oncology: a focus on cardiotoxicity. J Eur Cardiol Rev. 2018;13(1):64.
Ewer S, Lippman SM. Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity by Michael. J Clin Oncol. 2005;23:2900–2.
Ewer MS, et al. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23(31):7820–6.
Larsen CM, Mulvagh SL. Cardio-oncology: what you need to know now for clinical practice and echocardiography. Echo Res. 2017;4(1):R33–41.
Shah DR, Shah RR, Morganroth J. Tyrosine kinase inhibitors: their on-target toxicities as potential indicators of efficacy. Drug Saf. 2013;36(6):413–26.
Gupta R, Maitland ML. Sunitinib, hypertension, and heart failure: a model for kinase inhibitor-mediated cardiotoxicity. Curr Hypertens Rep. 2011;13(6):430–5.
Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007;7(5):332–44.
Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol. 2008;9(10):747–58.
Houles T, Roux PP. Defining the role of the RSK isoforms. in cancer in Seminars in cancer biology. Elsevier; 2018.
Romeo Y, Zhang X, Roux PP. Regulation and function of the RSK family of protein kinases. Biochem J. 2012;441(2):553–69.
Carrière A, et al. Oncogenic MAPK signaling stimulates mTORC1 activity by promoting RSK-mediated raptor phosphorylation. Curr Biol. 2008;18(17):1269–77.
Sears R, et al. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes. 2000;14(19):2501–14.
Shahbazian D, et al. The mTOR/PI3K and MAPK pathways converge on eIF4B to control its phosphorylation and activity. EMBO J. 2006;25(12):2781–91.
Anjum R, et al. The tumor suppressor DAP kinase is a target of RSK-mediated survival signaling. Curr Biol. 2005;15(19):1762–7.
Bonni A, et al. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and-independent mechanisms. Science. 1999;286(5443):1358–62.
Fujita N, Sato S, Tsuruo T. Phosphorylation of p27Kip1 at threonine 198 by p90 ribosomal protein S6 kinases promotes its binding to 14-3-3 and cytoplasmic localization. J Biol Chem. 2003;278(49):49254–60.
David J-P, et al. Essential role of RSK2 in c-Fos–dependent osteosarcoma development. J Clin Investig. 2005;115(3):664–72.
Diehl JA, et al. Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12(22):3499–511.
Sears R, et al. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 2000;14(19):2501–14.
Chen R-H, et al. Phosphorylation of c-Fos at the C-terminus enhances its transforming activity. Oncogene. 1996;12(7):1493–502.
Murphy LO, et al. Molecular interpretation of ERK signal duration by immediate early gene products. Nat Cell Biol. 2002;4(8):556–64.
Larrea MD, et al. RSK1 drives p27Kip1 phosphorylation at T198 to promote RhoA inhibition and increase cell motility. Proc Natl Acad Sci. 2009;106(23):9268–73.
Wu CF, et al. RSK promotes G2/M transition through activating phosphorylation of Cdc25A and Cdc25B. Oncogene. 2014;33(18):2385–94.
Zhu J, Blenis J, Yuan J. Activation of PI3K/Akt and MAPK pathways regulates Myc-mediated transcription by phosphorylating and promoting the degradation of Mad1. Proc Natl Acad Sci. 2008;105(18):6584–9.
Brüning JC, et al. Ribosomal subunit kinase-2 is required for growth factor-stimulated transcription of the c-Fos gene. Proc Natl Acad Sci. 2000;97(6):2462–7.
Nandagopal N, Roux PP. Regulation of global and specific mRNA translation by the mTOR signaling pathway. Translation. 2015;3(1):e983402.
Rolfe M, et al. Activation of protein synthesis in cardiomyocytes by the hypertrophic agent phenylephrine requires the activation of ERK and involves phosphorylation of tuberous sclerosis complex 2 (TSC2). Biochem J. 2005;388(3):973–84.
Roux PP, et al. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc Natl Acad Sci. 2004;101(37):13489–94.
Wang X, et al. Evidence that the dephosphorylation of Ser535 in the∊-subunit of eukaryotic initiation factor (eIF) 2B is insufficient for the activation of eIF2B by insulin. Biochem J. 2002;367(2):475–81.
Shahbazian D, et al. Control of cell survival and proliferation by mammalian eukaryotic initiation factor 4B. Mol Cell Biol. 2010;30(6):1478–85.
Galan JA, et al. Phosphoproteomic analysis identifies the tumor suppressor PDCD4 as a RSK substrate negatively regulated by 14-3-3. Proc Natl Acad Sci. 2014;111(29):E2918–27.
Cuesta R, Holz MK. RSK-mediated down-regulation of PDCD4 is required for proliferation, survival, and migration in a model of triple-negative breast cancer. Oncotarget. 2016;7(19):27567.
Roux PP, et al. RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J Biol Chem. 2007;282(19):14056–64.
Bonni A, et al. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286(5443):1358–62.
Shimamura A, et al. Rsk1 mediates a MEK–MAP kinase cell survival signal. Curr Biol. 2000;10(3):127–35.
Bialik S, Kimchi A. DAP-kinase as a target for drug design in cancer and diseases associated with accelerated cell death. in Seminars in cancer biology. Elsevier; 2004.
Buck M, et al. C/EBPβ phosphorylation by RSK creates a functional XEXD caspase inhibitory box critical for cell survival. Mol Cell. 2001;8(4):807–16.
Xing J, Ginty DD, Greenberg ME. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science. 1996;273(5277):959–63.
Doehn U, et al. RSK is a principal effector of the RAS-ERK pathway for eliciting a coordinate promotile/invasive gene program and phenotype in epithelial cells. Mol Cell. 2009;35(4):511–22.
Gawecka JE, et al. RSK2 protein suppresses integrin activation and fibronectin matrix assembly and promotes cell migration. J Biol Chem. 2012;287(52):43424–37.
Woo MS, et al. Ribosomal S6 kinase (RSK) regulates phosphorylation of filamin A on an important regulatory site. Mol Cell Biol. 2004;24(7):3025–35.
Tanimura S, et al. SH3P2 is a negative regulator of cell motility whose function is inhibited by ribosomal S6 kinase-mediated phosphorylation. Genes Cells. 2011;16(5):514–26.
Chen C, et al. Suppression of DNA-damage checkpoint signaling by Rsk-mediated phosphorylation of Mre11. Proc Natl Acad Sci. 2013;110(51):20605–10.
Ray-David H, et al. RSK promotes G2 DNA damage checkpoint silencing and participates in melanoma chemoresistance. Oncogene. 2013;32(38):4480–9.
Ludwik KA, et al. Development of a RSK inhibitor as a novel therapy for triple-negative breast cancer. Mol Cancer Ther. 2016;15(11):2598–608.
Kosnopfel C, et al. Inhibition of p90 ribosomal S6 kinases disrupts melanoma cell growth and immune evasion. J Exp Clin Cancer Res. 2023;42(1):175.
Takeishi Y, et al. Activation of mitogen-activated protein kinases and p90 ribosomal S6 kinase in failing human hearts with dilated cardiomyopathy. Cardiovasc Res. 2002;53(1):131–7.
Takeishi Y, et al. Differential regulation of p90 ribosomal S6 kinase and big mitogen–activated protein kinase 1 by ischemia/reperfusion and oxidative stress in perfused guinea pig hearts. Circ Res. 1999;85(12):1164–72.
Jaballah M, et al. Na+/H+ exchanger isoform 1 induced cardiomyocyte hypertrophy involves activation of p90 ribosomal s6 kinase. PLoS ONE. 2015;10(4):e0122230.
Xue J, et al. Elevated myocardial Na+/H+ exchanger isoform 1 activity elicits gene expression that leads to cardiac hypertrophy. Physiol Genom. 2010;42(3):374–83.
Lin L, White SA, Hu K. Role of p90RSK in kidney and other diseases. Int J Mol Sci. 2019;20(4):972.
Mentzer RM Jr, et al. Sodium-hydrogen exchange inhibition by cariporide to reduce the risk of ischemic cardiac events in patients undergoing coronary artery bypass grafting: results of the EXPEDITION study. Ann Thorac Surg. 2008;85(4):1261–70.
Amirak E, et al. p90 Ribosomal S6 kinases play a significant role in early gene regulation in the cardiomyocyte response to Gq-protein-coupled receptor stimuli, endothelin-1 and α1-adrenergic receptor agonists. Biochem J. 2013;450(2):351–63.
Yamaguchi N, et al. Dysfunctional ryanodine receptor and cardiac hypertrophy: role of signaling molecules. Am J Physiol-Heart. 2011;300(6):H2187–95.
He Q, et al. PKA, Rap1, ERK1/2, and p90RSK mediate PGE2 and EP4 signaling in neonatal ventricular myocytes. Am J Physiol-Heart. 2010;298(1):H136–43.
Lu Z, et al. Reactive oxygen species-induced activation of p90 ribosomal S6 kinase prolongs cardiac repolarization through inhibiting outward K+ channel activity. Circ Res. 2008;103(3):269–78.
Le N-T, et al. Flow signaling and atherosclerosis. Cell Mol Life Sci. 2017;74(10):1835–58.
Li J, et al. Anchored p90 ribosomal S6 kinase 3 is required for cardiac myocyte hypertrophy. Circ Res. 2013;112(1):128–39.
Paez-Mayorga J, et al. Ponatinib activates an inflammatory response in endothelial cells via ERK5 SUMOylation. Front Cardiovasc Med. 2018;5:125.
Uitdehaag JC, et al. Comparison of the cancer gene targeting and biochemical selectivities of all targeted kinase inhibitors approved for clinical use. PLoS One. 2014;9(3):e92146.
Karaman MW, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26(1):127–32.
Hasinoff BB, Patel D, O’Hara KA. Mechanisms of myocyte cytotoxicity induced by the multiple receptor tyrosine kinase inhibitor sunitinib. Mol Pharmacol. 2008;74(6):1722–8.
Yang WS, et al. RSK1 and RSK2 serine/threonine kinases regulate different transcription programs in cancer. Front Cell Dev Biol. 2022;10:1015665.
Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75(1):50–83.
Zhang L, et al. In vivo antitumor and antimetastatic activity of sunitinib in preclinical neuroblastoma mouse model. Neoplasia. 2009;11(5):426–35.
Kerkela R, et al. Sunitinib-induced cardiotoxicity is mediated by off-target inhibition of AMP-activated protein kinase. Clin Transl Sci. 2009;2(1):15–25.
Deng L, et al. Luteolin, a novel p90 ribosomal S6 kinase inhibitor, suppresses proliferation and migration in leukemia cells. Oncol Lett. 2017;13(3):1370–8.
Suleiman M. The role of p90 ribosomal s6 kinase and autophagy in sunitinib and ponatinib-induced cardiotoxicity. 2019. Master's thesis.
Martinez EC, et al. RSK3: a regulator of pathological cardiac remodeling. IUBMB Life. 2015;67(5):331–7.
Hurtado-de-Mendoza D, Loaiza-Bonilla A, Bonilla-Reyes PA, Tinoco G, Alcorta R. Cardio-oncology: cancer therapy-related cardiovascular complications in a molecular targeted era: new concepts and perspectives. Cureus. 2017;9(5).
Aparicio-Gallego G, et al. New insights into molecular mechanisms of sunitinib-associated side effects. Mol Cancer Ther. 2011;10(12):2215–23.
Motzer RJ, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–24.
Graves A, Hessamodini H, Wong G, Lim WH. Metastatic renal cell carcinoma: update on epidemiology, genetics, and therapeutic modalities. ImmunoTargets and Therapy. 2013;73–90.
Sayed-Ahmed MM, et al. Carnitine supplementation attenuates sunitinib-induced inhibition of amp-activated protein kinase downstream signals in cardiac tissues. Cardiovasc Toxicol. 2019;19(4):344–56.
Hao Z, Sadek I. Sunitinib: the antiangiogenic effects and beyond. OncoTargets. 2016;9:5495.
Faivre S, et al. Molecular basis for sunitinib efficacy and future clinical development. Nat Rev Drug Discov. 2007;6(9):734–45.
Rossari F, et al. Past, present, and future of Bcr-Abl inhibitors: from chemical development to clinical efficacy. J Hematol. 2018;11(1):1–14.
Pophali PA, Patnaik MM. The role of new tyrosine kinase inhibitors in chronic myeloid leukemia. Cancer J. 2016;22(1):40.
Tan FH, et al. Ponatinib: a novel multi-tyrosine kinase inhibitor against human malignancies. OncoTargets. 2019;12:635.
Huang W-S, et al. Discovery of 3-[2-(imidazo [1, 2-b] pyridazin-3-yl) ethynyl]-4-methyl-N-{4-[(4-methylpiperazin-1-yl) methyl]-3-(trifluoromethyl) phenyl} benzamide (AP24534), a potent, orally active pan-inhibitor of breakpoint cluster region-abelson (BCR-ABL) kinase including the T315I gatekeeper mutant. J Med Chem. 2010;53(12):4701–19.
Dao K-HT, Tyner JW. Next-generation medicine: combining BCR-ABL and Hedgehog-targeted therapies. Clin Cancer Res. 2013;19(6):1309–11.
Sandhu H, et al. Attenuation of sunitinib-induced cardiotoxicity through the A3 adenosine receptor activation. Eur J Pharmacol. 2017;814:95–105.
Lara R, Seckl MJ, Pardo OE. The p90 RSK family members: common functions and isoform specificity. Can Res. 2013;73(17):5301–8.
Sulzmaier FJ, Ramos JW. RSK isoforms in cancer cell invasion and metastasis. Can Res. 2013;73(20):6099–105.
Woo MS, et al. Ribosomal S6 kinase (RSK) regulates phosphorylation of filamin A on an important regulatory site. Mol Cell Biol. 2004;24(7):3025–35.
Gawecka JE, et al. RSK2 protein suppresses integrin activation and fibronectin matrix assembly and promotes cell migration. Mol Cell Biol. 2012;287(52):43424–37.
Abdulrahman N, et al. Inhibition of p90 ribosomal S6 kinase attenuates cell migration and proliferation of the human lung adenocarcinoma through phospho-GSK-3β and osteopontin. Mol Cell Biochem. 2016;418(1–2):21–9.
Poomakkoth N, et al. p90 ribosomal S6 kinase: a potential therapeutic target in lung cancer. J Transl Med. 2016;14:14.
Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: crosstalk and compensation. Trends Biochem Sci. 2011;36(6):320–8.
Hartmann S, Ridley AJ, Lutz S. The function of rho-associated kinases ROCK1 and ROCK2 in the pathogenesis of cardiovascular disease. Front Pharmacol. 2015;6:276.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Associate Editor Yihua Bei oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Suleiman, M., Al Najjar, A., Zakaria, Z.Z. et al. The Role of p90 Ribosomal S6 Kinase (RSK) in Tyrosine Kinase Inhibitor (TKI)-Induced Cardiotoxicity. J. of Cardiovasc. Trans. Res. 17, 334–344 (2024). https://doi.org/10.1007/s12265-023-10431-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-023-10431-4