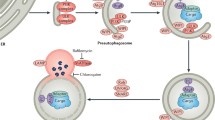
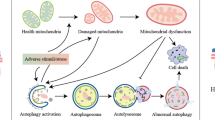
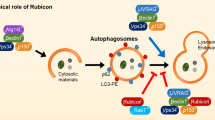
Abstract
Heart failure is a progressive disease with an annual mortality rate of about 10% and is the end-stage stage of various heart diseases, which places a huge socioeconomic burden on the healthcare system. The development of heart failure has received increasing attention as a potential way to improve the treatment of this disease. Many studies have shown that endoplasmic reticulum stress and autophagy play an important role in the occurrence and development of heart failure. With the in-depth study of endoplasmic reticulum stress and autophagy, both are considered promising targets for pharmacological interventions to treat heart failure, but the mechanism of heart failure between the two is not clear. This review will highlight the effects of endoplasmic reticulum stress, autophagy, and their interactions in the development and development of heart failure, thereby helping to provide direction for the future development of targeted therapies for patients with heart failure.
Clinical Relevance
This study explored the new targets for the treatment of heart failure: endoplasmic reticulum stress and autophagy. Targeted drug therapy for endoplasmic reticulum stress and autophagy is expected to provide a new intervention target for the treatment of heart failure.

Similar content being viewed by others
References
McDonagh TA, Metra M, Adamo M, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–726. https://doi.org/10.1093/eurheartj/ehab368.
Schwinger RHG. Pathophysiology of heart failure. Cardiovasc Diagn Ther. 2021;11(1):263–76. https://doi.org/10.21037/cdt-20-302.
Groenewegen A, Rutten FH, Mosterd A, et al. Epidemiology of heart failure. Eur J Heart Fail. 2020;22(8):1342–56. https://doi.org/10.1002/ejhf.1858.
Chen X, Zhang T, Zhang Y. Endoplasmic reticulum stress and autophagy in HIV-1-associated neurocognitive disorders. J Neurovirol. 2020;26(6):824–33. https://doi.org/10.1007/s13365-020-00906-4.
Zhang C, Syed TW, Liu R, et al. Role of endoplasmic reticulum stress, autophagy, and inflammation in cardiovascular disease. Front Cardiovascu Med. 2017;4:29. https://doi.org/10.3389/fcvm.2017.00029.
Ghosh R, Pattison JS. Macroautophagy and chaperone-mediated autophagy in heart failure: the known and the unknown. Oxid Med Cell Longev. 2018;2018:8602041. https://doi.org/10.1155/2018/8602041.
Hawes C, Kiviniemi P, Kriechbaumer V. The endoplasmic reticulum: a dynamic and well-connected organelle. J Integr Plant Biol. 2015;57(1):50–62. https://doi.org/10.1111/jipb.12297.
Kaushik S, Cuervo AM. Proteostasis and aging. Nat Med. 2015;21(12):1406–15. https://doi.org/10.1038/nm.4001.
Qi Z, Chen L. Endoplasmic reticulum stress and autophagy. Adv Exp Med Biol. 2019;1206:167–77. https://doi.org/10.1007/978-981-15-0602-4_8.
Cao T, Peng B, Zhou X, et al. Integrated signaling system under endoplasmic reticulum stress in eukaryotic microorganisms. Appl Microbiol Biotechnol. 2021;105(12):4805–18. https://doi.org/10.1007/s00253-021-11380-1.
Wang S, Binder P, Fang Q, et al. Endoplasmic reticulum stress in the heart: insights into mechanisms and drug targets. Br J Pharmacol. 2018;175(8):1293–304. https://doi.org/10.1111/bph.13888.
Ren J, Bi Y, Sowers JR, et al. Endoplasmic reticulum stress and unfolded protein response in cardiovascular diseases. Nature Reviews. Cardiology. 2021;18(7):499–521. https://doi.org/10.1038/s41569-021-00511-w.
Wang X, Xu L, Gillette TG, et al. The unfolded protein response in ischemic heart disease. J Mol Cell Cardiol. 2018;117:19–25. https://doi.org/10.1016/j.yjmcc.2018.02.013.
Jurkin J, Henkel T, Nielsen AF, et al. The mammalian tRNA ligase complex mediates splicing of XBP1 mRNA and controls antibody secretion in plasma cells. EMBO J. 2014;33(24):2922–36. https://doi.org/10.15252/embj.201490332.
Yücel SS, Stelzer W, Lorenzoni A, et al. The metastable XBP1u transmembrane domain defines determinants for intramembrane proteolysis by signal peptide peptidase. Cell Rep. 2019;26(11):3087–3099.e11. https://doi.org/10.1016/j.celrep.2019.02.057.
Rozpedek W, Pytel D, Mucha B, et al. The role of the PERK/eIF2α/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Curr Mol Med. 2016;16(6):533–44. https://doi.org/10.2174/1566524016666160523143937.
Fusakio ME, Willy JA, Wang Y, et al. Transcription factor ATF4 directs basal and stress-induced gene expression in the unfolded protein response and cholesterol metabolism in the liver. Mol Biol Cell. 2016;27(9):1536–51. https://doi.org/10.1091/mbc.E16-01-0039.
Papaioannou A, Higa A, Jégou G, et al. Alterations of EDEM1 functions enhance ATF6 pro-survival signaling. FEBS J. 2018;285(22):4146–64. https://doi.org/10.1111/febs.14669.
Okada K, Minamino T, Tsukamoto Y, et al. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation. 2004;110(6):705–12. https://doi.org/10.1161/01.CIR.0000137836.95625.D4.
Ni L, Zhou C, Duan Q, et al. β-AR blockers suppresses ER stress in cardiac hypertrophy and heart failure. PloS One. 2011;6(11):e27294. https://doi.org/10.1371/journal.pone.0027294.
Ortega A, Roselló-Lletí E, Tarazón E, et al. Endoplasmic reticulum stress induces different molecular structural alterations in human dilated and ischemic cardiomyopathy. PloS One. 2014;9(9):e107635. https://doi.org/10.1371/journal.pone.0107635.
Abushouk AI, Ismail A, Salem AMA, et al. Cardioprotective mechanisms of phytochemicals against doxorubicin-induced cardiotoxicity. Biomed Pharmacother. 2017;90:935–46. https://doi.org/10.1016/j.biopha.2017.04.033.
Sabzichi M, Mohammadian J, Ghorbani M, et al. Fabrication of all-trans-retinoic acid-loaded biocompatible precirol: a strategy for escaping dose-dependent side effects of doxorubicin. Colloids Surf B Biointerfaces. 2017;159:620–8. https://doi.org/10.1016/j.colsurfb.2017.08.030.
Maiuolo J, Bava I, Carresi C, et al. The effects of bergamot polyphenolic fraction, Cynara cardunculus, and Olea europea L. extract on doxorubicin-induced cardiotoxicity. Nutrients. 2021;13(7):2158. https://doi.org/10.3390/nu13072158.
Gao G, Jiang S, Ge L, et al. Atorvastatin improves doxorubicin-induced cardiac dysfunction by modulating Hsp70, Akt, and MAPK signaling pathways. J Cardiovasc Pharmacol. 2019;73(4):223–31. https://doi.org/10.1097/FJC.0000000000000646.
Schiattarella GG, Altamirano F, Kim SY, et al. Xbp1s-FoxO1 axis governs lipid accumulation and contractile performance in heart failure with preserved ejection fraction. Nature. Communications. 2021;12(1):1684. https://doi.org/10.1038/s41467-021-21931-9.
Sawada T, Minamino T, Fu HY, et al. X-box binding protein 1 regulates brain natriuretic peptide through a novel AP1/CRE-like element in cardiomyocytes. J Mol Cell Cardiol. 2010;48(6):1280–9. https://doi.org/10.1016/j.yjmcc.2010.02.004.
Binder P, Wang S, Radu M, et al. Pak2 as a novel therapeutic target for cardioprotective endoplasmic reticulum stress response. Circ Res. 2019;124(5):696–711. https://doi.org/10.1161/CIRCRESAHA.118.312829.
Wang J, Hu X, Jiang H. ER stress-induced apoptosis: a novel therapeutic target in heart failure. Int J Cardiol. 2014;177(2):564–5. https://doi.org/10.1016/j.ijcard.2014.08.118.
Yao Y, Lu Q, Hu Z, et al. A non-canonical pathway regulates ER stress signaling and blocks ER stress-induced apoptosis and heart failure. Nat Commun. 2017;8(1):133. https://doi.org/10.1038/s41467-017-00171-w.
Ayala P, Montenegro J, Vivar R, et al. Attenuation of endoplasmic reticulum stress using the chemical chaperone 4-phenylbutyric acid prevents cardiac fibrosis induced by isoproterenol. Exp Mol Pathol. 2012;92(1):97–104. https://doi.org/10.1016/j.yexmp.2011.10.012.
Castillero E, Akashi H, Pendrak K, et al. Attenuation of the unfolded protein response and endoplasmic reticulum stress after mechanical unloading in dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2015;309(3):H459–70. https://doi.org/10.1152/ajpheart.00056.2015.
Fu HY, Okada K, Liao Y, et al. Ablation of C/EBP homologous protein attenuates endoplasmic reticulum-mediated apoptosis and cardiac dysfunction induced by pressure overload. Circulation. 2010;122(4):361–9. https://doi.org/10.1161/CIRCULATIONAHA.109.917914.
Chang W-T, Lin Y-W, Ho C-H, et al. Dapagliflozin suppresses ER stress and protects doxorubicin-induced cardiotoxicity in breast cancer patients. Arch Toxicol. 2021;95(2):659–71. https://doi.org/10.1007/s00204-020-02951-8.
Ren F-F, Xie Z-Y, Jiang Y-N, et al. Dapagliflozin attenuates pressure overload-induced myocardial remodeling in mice via activating SIRT1 and inhibiting endoplasmic reticulum stress. Acta Pharmacol Sin. 2022;43(7):1721–32. https://doi.org/10.1038/s41401-021-00805-2.
Monceaux K, Gressette M, Karoui A, et al. Ferulic acid, pterostilbene, and tyrosol protect the heart from ER-stress-induced injury by activating SIRT1-dependent deacetylation of eIF2α. Int J Mol Sci. 2022;23(12):6628. https://doi.org/10.3390/ijms23126628.
Ichimiya T, Yamakawa T, Hirano T, et al. Autophagy and autophagy-related diseases: a review. Int J Mol Sci. 2020;21(23):8974. https://doi.org/10.3390/ijms21238974.
Ariosa AR, Lahiri V, Lei Y, et al. A perspective on the role of autophagy in cancer. Biochim Biophys Acta-Mol Basis Dis. 2021;1867(12):166262. https://doi.org/10.1016/j.bbadis.2021.166262.
Wang Y, Zhang H. Regulation of autophagy by mTOR signaling pathway. Adv Exp Med Biol. 2019;1206:67–83. https://doi.org/10.1007/978-981-15-0602-4_3.
Baudot AD, Wang VM-Y, Leach JD, et al. Glycan degradation promotes macroautophagy. Proc Natl Acad Sci U S A. 2022;119(26):e2111506119. https://doi.org/10.1073/pnas.2111506119.
Klionsky DJ, Schulman BA. Dynamic regulation of macroautophagy by distinctive ubiquitin-like proteins. Nat Struct Mol Biol. 2014;21(4):336–45. https://doi.org/10.1038/nsmb.2787.
Wu X, Liu Z, Yu X-Y, et al. Autophagy and cardiac diseases: therapeutic potential of natural products. Med Res Rev. 2021;41(1):314–41. https://doi.org/10.1002/med.21733.
Sica V, Galluzzi L, Bravo-San Pedro JM, et al. Organelle-specific initiation of autophagy. Mol Cell. 2015;59(4):522–39. https://doi.org/10.1016/j.molcel.2015.07.021.
Russell RC, Tian Y, Yuan H, et al. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat Cell Biol. 2013;15(7):741–50. https://doi.org/10.1038/ncb2757.
Ravikumar B, Sarkar S, Davies JE, et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90(4):1383–435. https://doi.org/10.1152/physrev.00030.2009.
Wang Z, Miao G, Xue X, et al. The Vici syndrome protein EPG5 is a Rab7 effector that determines the fusion specificity of autophagosomes with late endosomes/lysosomes. Mol Cell. 2016;63(5):781–95. https://doi.org/10.1016/j.molcel.2016.08.021.
Takáts S, Pircs K, Nagy P, et al. Interaction of the HOPS complex with Syntaxin 17 mediates autophagosome clearance in Drosophila. Mol Biol Cell. 2014;25(8):1338–54. https://doi.org/10.1091/mbc.e13-08-0449.
Jiang P, Nishimura T, Sakamaki Y, et al. The HOPS complex mediates autophagosome-lysosome fusion through interaction with syntaxin 17. Mol Biol Cell. 2014;25(8):1327–37. https://doi.org/10.1091/mbc.e13-08-0447.
Winkle AJ, Nassal DM, Shaheen R, et al. Emerging therapeutic targets for cardiac hypertrophy. Expert Opin Ther Targets. 2022;26(1):29–40. https://doi.org/10.1080/14728222.2022.2031974.
Burchfield JS, Xie M, Hill JA. Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation. 2013;128(4):388–400. https://doi.org/10.1161/CIRCULATIONAHA.113.001878.
Shah AK, Bhullar SK, Elimban V, et al. Oxidative stress as a mechanism for functional alterations in cardiac hypertrophy and heart failure. Antioxidants (Basel, Switzerland). 2021;10(6):931. https://doi.org/10.3390/antiox10060931.
Oyabu J, Yamaguchi O, Hikoso S, et al. Autophagy-mediated degradation is necessary for regression of cardiac hypertrophy during ventricular unloading. Biochem Biophys Res Commun. 2013;441(4):787–92. https://doi.org/10.1016/j.bbrc.2013.10.135.
Gatica D, Chiong M, Lavandero S, et al. The role of autophagy in cardiovascular pathology. Cardiovasc Res. 2022;118(4):934–50. https://doi.org/10.1093/cvr/cvab158.
Yan X, Zhang Y-L, Zhang L, et al. Gallic acid suppresses cardiac hypertrophic remodeling and heart failure. Mol Nutr Food Res. 2019;63(5):e1800807. https://doi.org/10.1002/mnfr.201800807.
Czubryt MP, Hale TM. Cardiac fibrosis: pathobiology and therapeutic targets. Cell Signal. 2021;85:110066. https://doi.org/10.1016/j.cellsig.2021.110066.
Wang L, Yuan D, Zheng J, et al. Chikusetsu saponin IVa attenuates isoprenaline-induced myocardial fibrosis in mice through activation autophagy mediated by AMPK/mTOR/ULK1 signaling. Phytomed: Int J Phytotherapy and Phytopharmacol. 2019;58:152764. https://doi.org/10.1016/j.phymed.2018.11.024.
Santulli G. Cardioprotective effects of autophagy: eat your heart out, heart failure! Sci Transl Med. 2018;10(443):eaau0462. https://doi.org/10.1126/scitranslmed.aau0462.
Guo X, Zhang Y, Lu C, et al. Protective effect of hyperoside on heart failure rats via attenuating myocardial apoptosis and inducing autophagy. Biosci Biotechnol Biochem. 2020;84(4):714–24. https://doi.org/10.1080/09168451.2019.1685369.
Zhou W-W, Dai C, Liu W-Z, et al. Gentianella acuta improves TAC-induced cardiac remodelling by regulating the Notch and PI3K/Akt/FOXO1/3 pathways. Biomed Pharmacother. 2022;154:113564. https://doi.org/10.1016/j.biopha.2022.113564.
Chen X, Xu S, Zhao C, et al. Role of TLR4/NADPH oxidase 4 pathway in promoting cell death through autophagy and ferroptosis during heart failure. Biochem Biophys Res Commun. 2019;516(1):37–43. https://doi.org/10.1016/j.bbrc.2019.06.015.
Gao T, Zhang S-P, Wang J-F, et al. TLR3 contributes to persistent autophagy and heart failure in mice after myocardial infarction. J Cell Mol Med. 2018;22(1):395–408. https://doi.org/10.1111/jcmm.13328.
Zaffagnini G, Martens S. Mechanisms of selective autophagy. J Mol Biol. 2016;428(9 Pt A):1714–24. https://doi.org/10.1016/j.jmb.2016.02.004.
Li W, He P, Huang Y, et al. Selective autophagy of intracellular organelles: recent research advances. Theranostics. 2021;11(1):222–56. https://doi.org/10.7150/thno.49860.
Faruk MO, Ichimura Y, Komatsu M. Selective autophagy. Cancer Sci. 2021;112(10):3972–8. https://doi.org/10.1111/cas.15112.
Wang Y, Zhou L, Su W, et al. Selective inhibition of PKCβ2 restores ischemic postconditioning-mediated cardioprotection by modulating autophagy in diabetic rats. J Diabetes Res. 2020;2020:2408240. https://doi.org/10.1155/2020/2408240.
Chen H, Zhou J, Chen H, et al. Bmi-1-RING1B prevents GATA4-dependent senescence-associated pathological cardiac hypertrophy by promoting autophagic degradation of GATA4. Clin Transl Med. 2022;12(4):e574. https://doi.org/10.1002/ctm2.574.
Kouroku Y, Fujita E, Tanida I, et al. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007;14(2):230–9. https://doi.org/10.1038/sj.cdd.4401984.
Bhardwaj M, Leli NM, Koumenis C, et al. Regulation of autophagy by canonical and non-canonical ER stress responses. Semin Cancer Biol. 2020;66:116–28. https://doi.org/10.1016/j.semcancer.2019.11.007.
Pires Da Silva J, Monceaux K, Guilbert A, et al. SIRT1 protects the heart from ER stress-induced injury by promoting eEF2K/eEF2-dependent autophagy. Cells. 2020;9(2):426. https://doi.org/10.3390/cells9020426.
Xu Z-M, Li C-B, Liu Q-L, et al. Ginsenoside Rg1 prevents doxorubicin-induced cardiotoxicity through the inhibition of autophagy and endoplasmic reticulum stress in mice. Int J Mol Sci. 2018;19(11):3658. https://doi.org/10.3390/ijms19113658.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82104721), Tianjin Famous Traditional Chinese Medicine (Junping Zhang) Inheritance Studio Special Funding (Jin Wei Zhong [2020] No. 732), and QI HUANG Scholars (Junping Zhang) Special Funding (National Traditional Chinese Medicine People’s Education Letter [2021] grant number 203).
Author information
Authors and Affiliations
Contributions
Leilei Hu contributed to proposing ideas; Yingyu Xie and Junping Zhang were responsible for orientation; Leilei Hu and Dongjie Gao were responsible for writing—original draft preparation; Leilei Hu, Dongjie Gao, Mingyang Wang, Hao Lv, and Lu Lian were responsible for content modification; Leilei Hu, Hao Lv, and Yunjiao Wang were responsible for visualization; Junping Zhang was responsible for funding acquisition. All authors have agreed to the publication of this manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Research Involving Human Participants and/or Animals
No human/ animal studies were carried out by the authors for this article.
Informed Consent
Patient consent for publication is not required.
Consent to Participate
Patient and public involvement and patients and/or the public were not involved in the design, conduct, reporting, or dissemination plans of this research.
Competing Interests
The authors declare no competing interests.
Additional information
Associate Editor Junjie Xiao oversaw the review of this article
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, L., Gao, D., Lv, H. et al. Finding New Targets for the Treatment of Heart Failure: Endoplasmic Reticulum Stress and Autophagy. J. of Cardiovasc. Trans. Res. 16, 1349–1356 (2023). https://doi.org/10.1007/s12265-023-10410-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-023-10410-9