Abstract
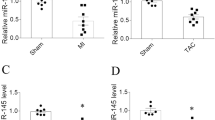
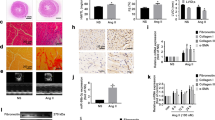
Increasing evidence has shown that microRNAs (miRNAs) participate in cardiac fibrosis. We aimed to elucidate the effect of miRNA miR-25-3p on cardiac fibrosis. MiRNA microarray was used to profile miRNAs in the myocardium of angiotensin-II (Ang-II)-infused mice. Effect of miR-25-3p on expression of fibrosis-related genes, including Col1a1, Col3a1, and Acta2, was investigated both in vitro and in vivo. MiR-25-3p was shown increased in the myocardium of Ang-II-infused mice and patients with heart failure. MiR-25-3p enhanced fibrosis-related gene expression in mouse cardiac fibroblasts (mCFs) and in the myocardium of Ang-II-infused mice. Dickkopf 3 (Dkk3) was identified as a target gene of miR-25-3p, and Dkk3 could ameliorate Smad3 activation and fibrosis-related gene expression via enhancing Smad7 expression in mCFs. Additionally, NF-κB signal was proven to mediate upregulation of miR-25-3p in cardiac fibrosis. Our findings suggest that miR-25-3p enhances cardiac fibrosis by suppressing Dkk3 to activate Smad3 and fibrosis-related gene expression.
Graphical abstract







Similar content being viewed by others
Abbreviations
- miR:
-
microRNA
- Ang-II:
-
Angiotensin-II
- Dkk3:
-
Dickkopf 3
- mCFs:
-
Mouse cardiac fibroblasts
- HF:
-
Heart failure
- TAC:
-
Transaortic constriction
- Smad7:
-
SMAD family member 7
- LV:
-
Left ventricular
- EF:
-
Ejection fraction
- FS:
-
Fractional shortening
- CVF:
-
Collagen volume fraction
- NMVCs:
-
Neonatal mouse ventricular cardiomyocytes
- RT-qPCR:
-
Quantitative reverse-transcription PCR
- FL:
-
Firefly luciferase
- RL:
-
Renilla luciferase
- ChIP:
-
Chromatin immunoprecipitation
- EdU:
-
5-Ethynyl-2′-deoxyuridine
- 3′-UTR:
-
3′-untranslation region
References
Dzeshka, M. S., Lip, G. Y., Snezhitskiy, V., & Shantsila, E. (2015). Cardiac fibrosis in patients with atrial fibrillation: mechanisms and clinical implications. Journal of the American College of Cardiology, 66, 943–959.
Fan, Z. Z., & Guan, J. J. (2016). Antifibrotic therapies to control cardiac fibrosis. Biomaterials Research, 20, 13.
Niehrs, C. (2006). Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene, 25, 7469–7481.
Monaghan, A. P., Kioschis, P., Wu, W., Zuniga, A., Bock, D., Poustka, A., et al. (1999). Dickkopf genes are coordinately expressed in mesodermal lineages. Mechanisms of Development, 87, 45–56.
Mallarino, R., Campas, O., Fritz, J. A., Burns, K. J., Weeks, O. G., Brenner, M. P., et al. (2012). Closely related bird species demonstrate flexibility between beak morphology and underlying developmental programs. Proceedings National Academy of Sciences United States of America, 109, 16,222–16,227.
Abarzua, F., Sakaguchi, M., Takaishi, M., Nasu, Y., Kurose, K., Ebara, S., et al. (2005). Adenovirus-mediated overexpression of REIC/Dkk-3 selectively induces apoptosis in human prostate cancer cells through activation of c-Jun-NH2-kinase. Cancer Research, 65, 9617–9622.
Kawano, Y., Kitaoka, M., Hamada, Y., Walker, M. M., Waxman, J., & Kypta, R. M. (2006). Regulation of prostate cell growth and morphogenesis by Dickkopf-3. Oncogene, 25, 6528–6537.
Papatriantafyllou, M., Moldenhauer, G., Ludwig, J., Tafuri, A., Garbi, N., Hollmann, G., et al. (2012). Dickkopf-3, an immune modulator in peripheral CD8 T-cell tolerance. Proceedings National Academy of Sciences United States of America, 109, 1631–1636.
Zhang, Y., Liu, Y., Zhu, X. H., Zhang, X. D., Jiang, D. S., Bian, Z. Y., et al. (2014). Dickkopf-3 attenuates pressure overload-induced cardiac remodeling. Cardiovascular Research, 102, 35–45.
Zhai, C. G., Xu, Y. Y., Tie, Y. Y., Zhang, Y., Chen, W. Q., Ji, X. P., et al. (2018). DKK3 overexpression attenuates cardiac hypertrophy and fibrosis in an angiotensin-perfused animal model by regulating the ADAM17/ACE2 and GSK-3beta/beta-catenin pathways. Journal of Molecular and Cellular Cardiology, 114, 243–252.
Miska, E. A. (2005). How microRNAs control cell division, differentiation and death. Current Opinion in Genetics & Development, 15, 563–568.
Li, Y., Liang, Y., Zhu, Y., Zhang, Y., & Bei, Y. (2018). Noncoding RNAs in cardiac hypertrophy. Journal of Cardiovascular Translational Research, 11, 439–449.
Henning, R. J. (2020). Cardiovascular exosomes and microRNAs in cardiovascular physiology and pathophysiology. Journal of Cardiovascular Translational Research. https://doi.org/10.1007/s12265-020-10040-5.
Bauersachs, J. (2010). Regulation of myocardial fibrosis by microRNAs. Journal of Cardiovascular Pharmacology, 56, 454–459.
Yang, Z. Z., Xiao, Z., Guo, H. M., Fang, X. H., Liang, J. N., Zhu, J. N., et al. (2019). Novel role of the clustered miR-23b-3p and miR-27b-3p in enhanced expression of fibrosis-associated genes by targeting TGFBR3 in atrial fibroblasts. Journal of Cellular and Molecular Medicine, 23, 3246–3256.
Zhu, W. S., Tang, C. M., Xiao, Z., Zhu, J. N., Lin, Q. X., Fu, Y. H., et al. (2016). Targeting EZH1 and EZH2 contributes to the suppression of fibrosis-associated genes by miR-214-3p in cardiac myofibroblasts. Oncotarget, 7, 78,331–78,342.
Jeong, D., Yoo, J., Lee, P., Kepreotis, S. V., Lee, A., Wahlquist, C., et al. (2018). miR-25 tough decoy enhances cardiac function in heart failure. Molecular Therapy, 26, 718–729.
Liu, Q., Wang, Y., Yang, T., & Wei, W. (2016). Protective effects of miR-25 against hypoxia/reoxygenation-induced fibrosis and apoptosis of H9c2 cells. International Journal of Molecular Medicine, 38, 1225–1234.
Genz, B., Coleman, M. A., Irvine, K. M., Kutasovic, J. R., Miranda, M., Gratte, F. D., et al. (2019). Overexpression of miRNA-25-3p inhibits Notch1 signaling and TGF-beta-induced collagen expression in hepatic stellate cells. Scientific Reports, 9, 8541.
Yuan, W. W., Tang, C. M., Zhu, W. S., Zhu, J. N., Lin, Q. X., Fu, Y. H., et al. (2016). CDK6 mediates the effect of attenuation of miR-1 on provoking cardiomyocyte hypertrophy. Molecular and Cellular Biochemistry, 412, 289–296.
Baylin, S. B., & Schuebel, K. E. (2007). Genomic biology: the epigenomic era opens. Nature, 448, 548–549.
Wahlquist, C., Jeong, D., Rojas-Muñoz, A., Kho, C., Lee, A., Mitsuyama, S., et al. (2014). Inhibition of miR-25 improves cardiac contractility in the failing heart. Nature, 508, 531–535.
Li, H., Xie, Y., Liu, Y., Qi, Y., Tang, C., Li, X., et al. (2018). Alteration in microRNA-25 expression regulate cardiac function via renin secretion. Experimental Cell Research, 365, 119–128.
Dirkx, E., Gladka, M. M., Philippen, L. E., Armand, A. S., Kinet, V., Leptidis, S., et al. (2013). Nfat and miR-25 cooperate to reactivate the transcription factor Hand2 in heart failure. Nature Cell Biology, 15, 1282–1293.
Wei, L. H., Huang, X. R., Zhang, Y., Li, Y. Q., Chen, H. Y., Yan, B. P., et al. (2013). Smad7 inhibits angiotensin II-induced hypertensive cardiac remodeling. Cardiovascular Research, 99, 665–673.
He, X., Gao, X., Peng, L., Wang, S., Zhu, Y., Ma, H., et al. (2011). Atrial fibrillation induces myocardial fibrosis through angiotensin II type 1 receptor-specific Arkadia-mediated downregulation of Smad7. Circulation Research, 108, 164–175.
Qi, H. P., Wang, Y., Zhang, Q. H., Guo, J., Li, L., Cao, Y. G., et al. (2015). Activation of peroxisome proliferator-activated receptor γ (PPARγ) through NF-κB/Brg1 and TGF-β1 pathways attenuates cardiac remodeling in pressure-overloaded rat hearts. Cellular Physiology and Biochemistry, 35, 899–912.
Cau, S. B., Guimaraes, D. A., Rizzi, E., Ceron, C. S., Gerlach, R. F., & Tanus-Santos, J. E. (2015). The nuclear factor kappa B inhibitor pyrrolidinedithiocarbamate prevents cardiac remodeling and matrix metalloproteinase-2 up-regulation in renovascular hypertension. Basic & Clinical Pharmacology & Toxicology, 117, 234–241.
Li, H., Xu, J. D., Fang, X. H., Zhu, J. N., Yang, J., Pan, R., et al. (2020). Circular RNA circRNA_000203 aggravates cardiac hypertrophy via suppressing miR-26b-5p and miR-140-3p binding to Gata4. Cardiovascular Research, 116, 1323–1334.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (82070254 and 81770264 to Z.-X. Shan), a High-level Hospital Construction Project of Guangdong Provincial People’s Hospital (DFJH201902 to Z.-X. Shan), and a Guangzhou Science and Technology Program project (202002030013 to X.-H. Fang).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Ni Zeng and Zhi-Xin Shan. Performed the experiments: Ni Zeng, Yi-Hong Wen, Rong Pan, An-Zhi Zhao, Jing Yang, and Yu-Min Yan. Managed data: Ni Zeng and Xian-Hong Fang. Analyzed the data: Yi-Hong Wen, Jie-Ning Zhu, and Zhi-Xin Shan. Contributed reagents/materials/analysis tools: Yu-Min Yan and Xian-Hong Fang. Funding acquisition: Xian-Hong Fang and Zhi-Xin Shan. Wrote the paper: Zhi-Xin Shan. All authors have read and agreed to the final version of manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that there are no conflicts of interest.
Additional information
Associate Editor Yihua Bei oversaw the review of this article
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 326 kb)
Rights and permissions
About this article
Cite this article
Zeng, N., Wen, YH., Pan, R. et al. Dickkopf 3: a Novel Target Gene of miR-25-3p in Promoting Fibrosis-Related Gene Expression in Myocardial Fibrosis. J. of Cardiovasc. Trans. Res. 14, 1051–1062 (2021). https://doi.org/10.1007/s12265-021-10116-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-021-10116-w