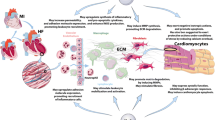
Abstract
In the infarcted myocardium, cardiomyocyte necrosis triggers an intense inflammatory reaction that not only is critical for cardiac repair, but also contributes to adverse remodeling and to the pathogenesis of heart failure. Both CC and CXC chemokines are markedly induced in the infarcted heart, bind to endothelial glycosaminoglycans, and regulate leukocyte trafficking and function. ELR+ CXC chemokines (such as CXCL8) control neutrophil infiltration, whereas CC chemokines (such as CCL2) mediate recruitment of mononuclear cells. Moreover, some members of the chemokine family (such as CXCL10 and CXCL12) may mediate leukocyte-independent actions, directly modulating fibroblast and vascular cell function. This review manuscript discusses our understanding of the role of the chemokines in regulation of injury, repair, and remodeling following myocardial infarction. Although several chemokines may be promising therapeutic targets in patients with myocardial infarction, clinical implementation of chemokine-based therapeutics is hampered by the broad effects of the chemokines in both injury and repair.



Similar content being viewed by others
References
Roth, G. A., Huffman, M. D., Moran, A. E., Feigin, V., Mensah, G. A., Naghavi, M., et al. (2015). Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation, 132(17), 1667–1678. https://doi.org/10.1161/CIRCULATIONAHA.114.008720.
Reimer, K. A., Lowe, J. E., Rasmussen, M. M., & Jennings, R. B. (1977). The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation, 56(5), 786–794.
Hjalmarson, A., Elmfeldt, D., Herlitz, J., Holmberg, S., Malek, I., Nyberg, G., et al. (1981). Effect on mortality of metoprolol in acute myocardial infarction. A double-blind randomised trial. Lancet, 2(8251), 823–827. https://doi.org/10.1016/s0140-6736(81)91101-6.
Pfeffer, M. A., Braunwald, E., Moye, L. A., Basta, L., Brown Jr., E. J., Cuddy, T. E., et al. (1992). Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med, 327(10), 669–677.
Effectiveness of intravenous thrombolytic treatment in acute myocardial infarction. Gruppo Italiano per lo Studio della Streptochinasi nell'Infarto Miocardico (GISSI) (1986). Lancet, 1(8478), 397-402.
Frangogiannis, N. G. (2014). The inflammatory response in myocardial injury, repair, and remodelling. Nature Reviews. Cardiology, 11(5), 255–265. https://doi.org/10.1038/nrcardio.2014.28.
Cavalera, M., & Frangogiannis, N. G. (2014). Targeting the chemokines in cardiac repair. Current Pharmaceutical Design, 20(12), 1971–1979. https://doi.org/10.2174/13816128113199990449.
Frangogiannis, N. G. (2007). Chemokines in ischemia and reperfusion. Thrombosis and Haemostasis, 97(5), 738–747.
Sokol, C. L., & Luster, A. D. (2015). The chemokine system in innate immunity. Cold Spring Harbor Perspectives in Biology, 7(5). https://doi.org/10.1101/cshperspect.a016303.
Kufareva, I., Salanga, C. L., & Handel, T. M. (2015). Chemokine and chemokine receptor structure and interactions: implications for therapeutic strategies. Immunology and Cell Biology, 93(4), 372–383. https://doi.org/10.1038/icb.2015.15.
Ahuja, S. K., & Murphy, P. M. (1996). The CXC chemokines growth-regulated oncogene (GRO) alpha, GRObeta, GROgamma, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. The Journal of Biological Chemistry, 271(34), 20545–20550. https://doi.org/10.1074/jbc.271.34.20545.
Clark-Lewis, I., Kim, K. S., Rajarathnam, K., Gong, J. H., Dewald, B., Moser, B., et al. (1995). Structure-activity relationships of chemokines. Journal of Leukocyte Biology, 57(5), 703–711. https://doi.org/10.1002/jlb.57.5.703.
Zlotnik, A., Morales, J., & Hedrick, J. A. (1999). Recent advances in chemokines and chemokine receptors. Critical Reviews in Immunology, 19(1), 1–47.
Zlotnik, A., & Yoshie, O. (2012). The chemokine superfamily revisited. Immunity, 36(5), 705–716. https://doi.org/10.1016/j.immuni.2012.05.008.
Islam, S. A., Chang, D. S., Colvin, R. A., Byrne, M. H., McCully, M. L., Moser, B., et al. (2011). Mouse CCL8, a CCR8 agonist, promotes atopic dermatitis by recruiting IL-5+ T(H)2 cells. Nature Immunology, 12(2), 167–177. https://doi.org/10.1038/ni.1984.
Jung, S., & Littman, D. R. (1999). Chemokine receptors in lymphoid organ homeostasis. Current Opinion in Immunology, 11(3), 319–325.
Murphy, P. M. (1996). Chemokine receptors: structure, function and role in microbial pathogenesis. Cytokine & Growth Factor Reviews, 7(1), 47–64.
Weathington, N. M., van Houwelingen, A. H., Noerager, B. D., Jackson, P. L., Kraneveld, A. D., Galin, F. S., et al. (2006). A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nature Medicine, 12(3), 317–323.
Bernhagen, J., Krohn, R., Lue, H., Gregory, J. L., Zernecke, A., Koenen, R. R., et al. (2007). MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nature Medicine, 13(5), 587–596. https://doi.org/10.1038/nm1567.
Lacy, M., Kontos, C., Brandhofer, M., Hille, K., Groning, S., Sinitski, D., et al. (2018). Identification of an Arg-Leu-Arg tripeptide that contributes to the binding interface between the cytokine MIF and the chemokine receptor CXCR4. Scientific Reports, 8(1), 5171. https://doi.org/10.1038/s41598-018-23554-5.
Alampour-Rajabi, S., El Bounkari, O., Rot, A., Muller-Newen, G., Bachelerie, F., Gawaz, M., et al. (2015). MIF interacts with CXCR7 to promote receptor internalization, ERK1/2 and ZAP-70 signaling, and lymphocyte chemotaxis. The FASEB Journal, 29(11), 4497–4511. https://doi.org/10.1096/fj.15-273904.
Soppert, J., Kraemer, S., Beckers, C., Averdunk, L., Mollmann, J., Denecke, B., et al. (2018). Soluble CD74 reroutes MIF/CXCR4/AKT-mediated survival of cardiac myofibroblasts to necroptosis. Journal of the American Heart Association, 7(17), e009384. https://doi.org/10.1161/JAHA.118.009384.
Frangogiannis, N. G. (2012). Regulation of the inflammatory response in cardiac repair. Circulation Research, 110(1), 159–173. https://doi.org/10.1161/CIRCRESAHA.111.243162.
Chen, B., Huang, S., Su, Y., Wu, Y. J., Hanna, A., Brickshawana, A., et al. (2019). Macrophage Smad3 protects the infarcted heart, stimulating phagocytosis and regulating inflammation. Circulation Research, 125(1), 55–70. https://doi.org/10.1161/CIRCRESAHA.119.315069.
Shinde, A. V., & Frangogiannis, N. G. (2014). Fibroblasts in myocardial infarction: a role in inflammation and repair. Journal of Molecular and Cellular Cardiology, 70C, 74–82.
Kanisicak, O., Khalil, H., Ivey, M. J., Karch, J., Maliken, B. D., Correll, R. N., et al. (2016). Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nature Communications, 7, 12260. https://doi.org/10.1038/ncomms12260.
Kong, P., Shinde, A. V., Su, Y., Russo, I., Chen, B., Saxena, A., et al. (2018). Opposing actions of fibroblast and cardiomyocyte Smad3 signaling in the infarcted myocardium. Circulation, 137(7), 707–724. https://doi.org/10.1161/CIRCULATIONAHA.117.029622.
Frangogiannis, N. G., Michael, L. H., & Entman, M. L. (2000). Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb). Cardiovascular Research, 48(1), 89–100.
Fu, X., Khalil, H., Kanisicak, O., Boyer, J. G., Vagnozzi, R. J., Maliken, B. D., et al. (2018). Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. The Journal of Clinical Investigation, 128(5), 2127–2143. https://doi.org/10.1172/JCI98215.
Kakio, T., Matsumori, A., Ono, K., Ito, H., Matsushima, K., & Sasayama, S. (2000). Roles and relationship of macrophages and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in the ischemic and reperfused rat heart. Laboratory Investigation, 80(7), 1127–1136.
Zouggari, Y., Ait-Oufella, H., Bonnin, P., Simon, T., Sage, A. P., Guerin, C., et al. (2013). B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nature Medicine, 19(10), 1273–1280.
Kumar, A. G., Ballantyne, C. M., Michael, L. H., Kukielka, G. L., Youker, K. A., Lindsey, M. L., et al. (1997). Induction of monocyte chemoattractant protein-1 in the small veins of the ischemic and reperfused canine myocardium. Circulation, 95(3), 693–700.
Frangogiannis, N. G., Mendoza, L. H., Lewallen, M., Michael, L. H., Smith, C. W., & Entman, M. L. (2001). Induction and suppression of interferon-inducible protein 10 in reperfused myocardial infarcts may regulate angiogenesis. The FASEB Journal, 15(8), 1428–1430.
Mylonas, K. J., Turner, N. A., Bageghni, S. A., Kenyon, C. J., White, C. I., McGregor, K., et al. (2017). 11beta-HSD1 suppresses cardiac fibroblast CXCL2, CXCL5 and neutrophil recruitment to the heart post MI. The Journal of Endocrinology, 233(3), 315–327. https://doi.org/10.1530/JOE-16-0501.
Turner, N. A., Das, A., O'Regan, D. J., Ball, S. G., & Porter, K. E. (2011). Human cardiac fibroblasts express ICAM-1, E-selectin and CXC chemokines in response to proinflammatory cytokine stimulation. The International Journal of Biochemistry & Cell Biology, 43(10), 1450–1458. https://doi.org/10.1016/j.biocel.2011.06.008.
Zymek, P., Nah, D. Y., Bujak, M., Ren, G., Koerting, A., Leucker, T., et al. (2007). Interleukin-10 is not a critical regulator of infarct healing and left ventricular remodeling. Cardiovascular Research, 74(2), 313–322. https://doi.org/10.1016/j.cardiores.2006.11.028.
Muhlstedt, S., Ghadge, S. K., Duchene, J., Qadri, F., Jarve, A., Vilianovich, L., et al. (2016). Cardiomyocyte-derived CXCL12 is not involved in cardiogenesis but plays a crucial role in myocardial infarction. Journal of Molecular Medicine (Berlin, Germany), 94(9), 1005–1014. https://doi.org/10.1007/s00109-016-1432-1.
Segret, A., Rucker-Martin, C., Pavoine, C., Flavigny, J., Deroubaix, E., Chatel, M. A., et al. (2007). Structural localization and expression of CXCL12 and CXCR4 in rat heart and isolated cardiac myocytes. The Journal of Histochemistry and Cytochemistry, 55(2), 141–150. https://doi.org/10.1369/jhc.6A7050.2006.
Ban, K., Ikeda, U., Takahashi, M., Kanbe, T., Kasahara, T., & Shimada, K. (1994). Expression of intercellular adhesion molecule-1 on rat cardiac myocytes by monocyte chemoattractant protein-1. Cardiovascular Research, 28(8), 1258–1262.
Herzog, C., Lorenz, A., Gillmann, H. J., Chowdhury, A., Larmann, J., Harendza, T., et al. (2014). Thrombomodulin's lectin-like domain reduces myocardial damage by interfering with HMGB1-mediated TLR2 signalling. Cardiovascular Research, 101(3), 400–410. https://doi.org/10.1093/cvr/cvt275.
Andrassy, M., Volz, H. C., Igwe, J. C., Funke, B., Eichberger, S. N., Kaya, Z., et al. (2008). High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation, 117(25), 3216–3226.
Fiuza, C., Bustin, M., Talwar, S., Tropea, M., Gerstenberger, E., Shelhamer, J. H., et al. (2003). Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood, 101(7), 2652–2660. https://doi.org/10.1182/blood-2002-05-1300.
Rossini, A., Zacheo, A., Mocini, D., Totta, P., Facchiano, A., Castoldi, R., et al. (2008). HMGB1-stimulated human primary cardiac fibroblasts exert a paracrine action on human and murine cardiac stem cells. Journal of Molecular and Cellular Cardiology, 44(4), 683–693. https://doi.org/10.1016/j.yjmcc.2008.01.009.
Lugrin, J., Parapanov, R., Rosenblatt-Velin, N., Rignault-Clerc, S., Feihl, F., Waeber, B., et al. (2015). Cutting edge: IL-1alpha is a crucial danger signal triggering acute myocardial inflammation during myocardial infarction. Journal of Immunology, 194(2), 499–503. https://doi.org/10.4049/jimmunol.1401948.
Chen, C., Feng, Y., Zou, L., Wang, L., Chen, H. H., Cai, J. Y., et al. (2014). Role of extracellular RNA and TLR3-Trif signaling in myocardial ischemia-reperfusion injury. Journal of the American Heart Association, 3(1), e000683. https://doi.org/10.1161/JAHA.113.000683.
Ghigo, A., Franco, I., Morello, F., & Hirsch, E. (2014). Myocyte signalling in leucocyte recruitment to the heart. Cardiovascular Research, 102(2), 270–280. https://doi.org/10.1093/cvr/cvu030.
Dybdahl, B., Slordahl, S. A., Waage, A., Kierulf, P., Espevik, T., & Sundan, A. (2005). Myocardial ischaemia and the inflammatory response: release of heat shock protein 70 after myocardial infarction. Heart, 91(3), 299–304.
Newton, K., & Dixit, V. M. (2012). Signaling in innate immunity and inflammation. Cold Spring Harbor Perspectives in Biology, 4(3). https://doi.org/10.1101/cshperspect.a006049.
Oyama, J., Blais Jr., C., Liu, X., Pu, M., Kobzik, L., Kelly, R. A., et al. (2004). Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation, 109(6), 784–789. https://doi.org/10.1161/01.CIR.0000112575.66565.84.
Kaczorowski, D. J., Nakao, A., Mollen, K. P., Vallabhaneni, R., Sugimoto, R., Kohmoto, J., et al. (2007). Toll-like receptor 4 mediates the early inflammatory response after cold ischemia/reperfusion. Transplantation, 84(10), 1279–1287. https://doi.org/10.1097/01.tp.0000287597.87571.17.
Feng, Y., Zhao, H., Xu, X., Buys, E. S., Raher, M. J., Bopassa, J. C., et al. (2008). Innate immune adaptor MyD88 mediates neutrophil recruitment and myocardial injury after ischemia-reperfusion in mice. American Journal of Physiology. Heart and Circulatory Physiology, 295(3), H1311–H1318. https://doi.org/10.1152/ajpheart.00119.2008.
Ao, L., Zou, N., Cleveland Jr., J. C., Fullerton, D. A., & Meng, X. (2009). Myocardial TLR4 is a determinant of neutrophil infiltration after global myocardial ischemia: mediating KC and MCP-1 expression induced by extracellular HSC70. American Journal of Physiology. Heart and Circulatory Physiology, 297(1), H21–H28. https://doi.org/10.1152/ajpheart.00292.2009.
Methe, H., Kim, J. O., Kofler, S., Weis, M., Nabauer, M., & Koglin, J. (2005). Expansion of circulating Toll-like receptor 4-positive monocytes in patients with acute coronary syndrome. Circulation, 111(20), 2654–2661. https://doi.org/10.1161/CIRCULATIONAHA.104.498865.
Satoh, M., Shimoda, Y., Maesawa, C., Akatsu, T., Ishikawa, Y., Minami, Y., et al. (2006). Activated toll-like receptor 4 in monocytes is associated with heart failure after acute myocardial infarction. International Journal of Cardiology, 109(2), 226–234. https://doi.org/10.1016/j.ijcard.2005.06.023.
Arslan, F., Smeets, M. B., O'Neill, L. A., Keogh, B., McGuirk, P., Timmers, L., et al. (2010). Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation, 121(1), 80–90.
Wang, J. W., Fontes, M. S. C., Wang, X., Chong, S. Y., Kessler, E. L., Zhang, Y. N., et al. (2017). Leukocytic Toll-like receptor 2 deficiency preserves cardiac function and reduces fibrosis in sustained pressure overload. Scientific Reports, 7(1), 9193. https://doi.org/10.1038/s41598-017-09451-3.
Lu, C., Ren, D., Wang, X., Ha, T., Liu, L., Lee, E. J., et al. (2014). Toll-like receptor 3 plays a role in myocardial infarction and ischemia/reperfusion injury. Biochimica et Biophysica Acta, 1842(1), 22–31.
de Kleijn, D. P. V., Chong, S. Y., Wang, X., Yatim, S., Fairhurst, A. M., Vernooij, F., et al. (2019). Toll-like receptor 7 deficiency promotes survival and reduces adverse left ventricular remodelling after myocardial infarction. Cardiovascular Research, 115(12), 1791–1803. https://doi.org/10.1093/cvr/cvz057.
Feng, Y., Chen, H., Cai, J., Zou, L., Yan, D., Xu, G., et al. (2015). Cardiac RNA induces inflammatory responses in cardiomyocytes and immune cells via Toll-like receptor 7 signaling. J Biol Chem, 290(44), 26688-26698, doi:10.1074/jbc. M115.661835.
Frantz, S., Kelly, R. A., & Bourcier, T. (2001). Role of TLR-2 in the activation of nuclear factor kappaB by oxidative stress in cardiac myocytes. The Journal of Biological Chemistry, 276(7), 5197–5203. https://doi.org/10.1074/jbc.M009160200.
Yang, R., Song, Z., Wu, S., Wei, Z., Xu, Y., & Shen, X. (2018). Toll-like receptor 4 contributes to a myofibroblast phenotype in cardiac fibroblasts and is associated with autophagy after myocardial infarction in a mouse model. Atherosclerosis, 279, 23–31. https://doi.org/10.1016/j.atherosclerosis.2018.10.018.
Zhang, W., Lavine, K. J., Epelman, S., Evans, S. A., Weinheimer, C. J., Barger, P. M., et al. (2015). Necrotic myocardial cells release damage-associated molecular patterns that provoke fibroblast activation in vitro and trigger myocardial inflammation and fibrosis in vivo. Journal of the American Heart Association, 4(6), e001993. https://doi.org/10.1161/JAHA.115.001993.
Li, Y., Si, R., Feng, Y., Chen, H. H., Zou, L., Wang, E., et al. (2011). Myocardial ischemia activates an injurious innate immune signaling via cardiac heat shock protein 60 and Toll-like receptor 4. The Journal of Biological Chemistry, 286(36), 31308–31319.
Ritchie, M. E. (1998). Nuclear factor-kappaB is selectively and markedly activated in humans with unstable angina pectoris. Circulation, 98(17), 1707–1713. https://doi.org/10.1161/01.cir.98.17.1707.
Chandrasekar, B., Smith, J. B., & Freeman, G. L. (2001). Ischemia-reperfusion of rat myocardium activates nuclear factor-KappaB and induces neutrophil infiltration via lipopolysaccharide-induced CXC chemokine. Circulation, 103(18), 2296–2302. https://doi.org/10.1161/01.cir.103.18.2296.
Chandrasekar, B., & Freeman, G. L. (1997). Induction of nuclear factor kappaB and activation protein 1 in postischemic myocardium. FEBS Letters, 401(1), 30–34. https://doi.org/10.1016/s0014-5793(96)01426-3.
Onai, Y., Suzuki, J., Kakuta, T., Maejima, Y., Haraguchi, G., Fukasawa, H., et al. (2004). Inhibition of IkappaB phosphorylation in cardiomyocytes attenuates myocardial ischemia/reperfusion injury. Cardiovascular Research, 63(1), 51–59. https://doi.org/10.1016/j.cardiores.2004.03.002.
Li, H. L., Zhuo, M. L., Wang, D., Wang, A. B., Cai, H., Sun, L. H., et al. (2007). Targeted cardiac overexpression of A20 improves left ventricular performance and reduces compensatory hypertrophy after myocardial infarction. Circulation, 115(14), 1885–1894. https://doi.org/10.1161/CIRCULATIONAHA.106.656835.
Timmers, L., van Keulen, J. K., Hoefer, I. E., Meijs, M. F., van Middelaar, B., den Ouden, K., et al. (2009). Targeted deletion of nuclear factor kappaB p50 enhances cardiac remodeling and dysfunction following myocardial infarction. Circulation Research, 104(5), 699–706. https://doi.org/10.1161/CIRCRESAHA.108.189746.
Frantz, S., Tillmanns, J., Kuhlencordt, P. J., Schmidt, I., Adamek, A., Dienesch, C., et al. (2007). Tissue-specific effects of the nuclear factor kappaB subunit p50 on myocardial ischemia-reperfusion injury. The American Journal of Pathology, 171(2), 507–512. https://doi.org/10.2353/ajpath.2007.061042.
Pinckard, R. N., Olson, M. S., Giclas, P. C., Terry, R., Boyer, J. T., & O'Rourke, R. A. (1975). Consumption of classical complement components by heart subcellular membranes in vitro and in patients after acute myocardial infarction. The Journal of Clinical Investigation, 56(3), 740–750. https://doi.org/10.1172/JCI108145.
Hill, J. H., & Ward, P. A. (1971). The phlogistic role of C3 leukotactic fragments in myocardial infarcts of rats. The Journal of Experimental Medicine, 133(4), 885–900. https://doi.org/10.1084/jem.133.4.885.
Czermak, B. J., Sarma, V., Bless, N. M., Schmal, H., Friedl, H. P., & Ward, P. A. (1999). In vitro and in vivo dependency of chemokine generation on C5a and TNF-alpha. Journal of Immunology, 162(4), 2321–2325.
Kilgore, K. S., Park, J. L., Tanhehco, E. J., Booth, E. A., Marks, R. M., & Lucchesi, B. R. (1998). Attenuation of interleukin-8 expression in C6-deficient rabbits after myocardial ischemia/reperfusion. Journal of Molecular and Cellular Cardiology, 30(1), 75–85. https://doi.org/10.1006/jmcc.1997.0573.
Maekawa, N., Wada, H., Kanda, T., Niwa, T., Yamada, Y., Saito, K., et al. (2002). Improved myocardial ischemia/reperfusion injury in mice lacking tumor necrosis factor-alpha. Journal of the American College of Cardiology, 39(7), 1229–1235. https://doi.org/10.1016/s0735-1097(02)01738-2.
Bujak, M., Dobaczewski, M., Chatila, K., Mendoza, L. H., Li, N., Reddy, A., et al. (2008). Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. The American Journal of Pathology, 173(1), 57–67. https://doi.org/10.2353/ajpath.2008.070974.
Saxena, A., Chen, W., Su, Y., Rai, V., Uche, O. U., Li, N., et al. (2013). IL-1 induces proinflammatory leukocyte infiltration and regulates fibroblast phenotype in the infarcted myocardium. Journal of Immunology, 191(9), 4838–4848. https://doi.org/10.4049/jimmunol.1300725.
Frangogiannis, N. G., Lindsey, M. L., Michael, L. H., Youker, K. A., Bressler, R. B., Mendoza, L. H., et al. (1998). Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation, 98(7), 699–710. https://doi.org/10.1161/01.cir.98.7.699.
Somasundaram, P., Ren, G., Nagar, H., Kraemer, D., Mendoza, L., Michael, L. H., et al. (2005). Mast cell tryptase may modulate endothelial cell phenotype in healing myocardial infarcts. The Journal of Pathology, 205(1), 102–111. https://doi.org/10.1002/path.1690.
Oynebraten, I., Bakke, O., Brandtzaeg, P., Johansen, F. E., & Haraldsen, G. (2004). Rapid chemokine secretion from endothelial cells originates from 2 distinct compartments. Blood, 104(2), 314–320. https://doi.org/10.1182/blood-2003-08-2891.
Detmers, P. A., Lo, S. K., Olsen-Egbert, E., Walz, A., Baggiolini, M., & Cohn, Z. A. (1990). Neutrophil-activating protein 1/interleukin 8 stimulates the binding activity of the leukocyte adhesion receptor CD11b/CD18 on human neutrophils. The Journal of Experimental Medicine, 171(4), 1155–1162. https://doi.org/10.1084/jem.171.4.1155.
Herter, J., & Zarbock, A. (2013). Integrin regulation during leukocyte recruitment. Journal of Immunology, 190(9), 4451–4457. https://doi.org/10.4049/jimmunol.1203179.
Knall, C., Worthen, G. S., & Johnson, G. L. (1997). Interleukin 8-stimulated phosphatidylinositol-3-kinase activity regulates the migration of human neutrophils independent of extracellular signal-regulated kinase and p38 mitogen-activated protein kinases. Proceedings of the National Academy of Sciences of the United States of America, 94(7), 3052–3057. https://doi.org/10.1073/pnas.94.7.3052.
Legler, D. F., & Thelen, M. (2018). New insights in chemokine signaling. F1000Res, 7, 95, doi:https://doi.org/10.12688/f1000research.13130.1.
Sotsios, Y., & Ward, S. G. (2000). Phosphoinositide 3-kinase: a key biochemical signal for cell migration in response to chemokines. Immunological Reviews, 177, 217–235.
Bujak, M., Dobaczewski, M., Gonzalez-Quesada, C., Xia, Y., Leucker, T., Zymek, P., et al. (2009). Induction of the CXC chemokine interferon-gamma-inducible protein 10 regulates the reparative response following myocardial infarction. Circulation Research, 105(10), 973–983. https://doi.org/10.1161/CIRCRESAHA.109.199471.
Tarzami, S. T., Miao, W., Mani, K., Lopez, L., Factor, S. M., Berman, J. W., et al. (2003). Opposing effects mediated by the chemokine receptor CXCR2 on myocardial ischemia-reperfusion injury: recruitment of potentially damaging neutrophils and direct myocardial protection. Circulation, 108(19), 2387–2392. https://doi.org/10.1161/01.CIR.0000093192.72099.9A.
Jarrah, A. A., Schwarskopf, M., Wang, E. R., LaRocca, T., Dhume, A., Zhang, S., et al. (2018). SDF-1 induces TNF-mediated apoptosis in cardiac myocytes. Apoptosis, 23(1), 79–91. https://doi.org/10.1007/s10495-017-1438-3.
Strieter, R. M., Polverini, P. J., Kunkel, S. L., Arenberg, D. A., Burdick, M. D., Kasper, J., et al. (1995). The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. The Journal of Biological Chemistry, 270(45), 27348–27357.
Kukielka, G. L., Smith, C. W., LaRosa, G. J., Manning, A. M., Mendoza, L. H., Daly, T. J., et al. (1995). Interleukin-8 gene induction in the myocardium after ischemia and reperfusion in vivo. The Journal of Clinical Investigation, 95(1), 89–103. https://doi.org/10.1172/JCI117680.
Ivey, C. L., Williams, F. M., Collins, P. D., Jose, P. J., & Williams, T. J. (1995). Neutrophil chemoattractants generated in two phases during reperfusion of ischemic myocardium in the rabbit. Evidence for a role for C5a and interleukin-8. The Journal of Clinical Investigation, 95(6), 2720–2728. https://doi.org/10.1172/JCI117974.
Zeilhofer, H. U., & Schorr, W. (2000). Role of interleukin-8 in neutrophil signaling. Current Opinion in Hematology, 7(3), 178–182.
Boyle Jr., E. M., Kovacich, J. C., Hebert, C. A., Canty Jr., T. G., Chi, E., Morgan, E. N., et al. (1998). Inhibition of interleukin-8 blocks myocardial ischemia-reperfusion injury. The Journal of Thoracic and Cardiovascular Surgery, 116(1), 114–121. https://doi.org/10.1016/S0022-5223(98)70249-1.
Oral, H., Kanzler, I., Tuchscheerer, N., Curaj, A., Simsekyilmaz, S., Sonmez, T. T., et al. (2013). CXC chemokine KC fails to induce neutrophil infiltration and neoangiogenesis in a mouse model of myocardial infarction. Journal of Molecular and Cellular Cardiology, 60, 1–7. https://doi.org/10.1016/j.yjmcc.2013.04.006.
Xie, Q., Sun, Z., Chen, M., Zhong, Q., Yang, T., & Yi, J. (2015). IL-8 up-regulates proliferative angiogenesis in ischemic myocardium in rabbits through phosphorylation of Akt/GSK-3beta(ser9) dependent pathways. International Journal of Clinical and Experimental Medicine, 8(8), 12498–12508.
Shetelig, C., Limalanathan, S., Hoffmann, P., Seljeflot, I., Gran, J. M., Eritsland, J., et al. (2018). Association of IL-8 with infarct size and clinical outcomes in patients With STEMI. Journal of the American College of Cardiology, 72(2), 187–198. https://doi.org/10.1016/j.jacc.2018.04.053.
Moser, B., & Loetscher, P. (2001). Lymphocyte traffic control by chemokines. Nature Immunology, 2(2), 123–128. https://doi.org/10.1038/84219.
Fiorina, P., Ansari, M. J., Jurewicz, M., Barry, M., Ricchiuti, V., Smith, R. N., et al. (2006). Role of CXC chemokine receptor 3 pathway in renal ischemic injury. J Am Soc Nephrol, 17(3), 716–723. https://doi.org/10.1681/ASN.2005090954.
Khan, I. A., MacLean, J. A., Lee, F. S., Casciotti, L., DeHaan, E., Schwartzman, J. D., et al. (2000). IP-10 is critical for effector T cell trafficking and host survival in Toxoplasma gondii infection. Immunity, 12(5), 483–494. https://doi.org/10.1016/s1074-7613(00)80200-9.
Shiraha, H., Glading, A., Gupta, K., & Wells, A. (1999). IP-10 inhibits epidermal growth factor-induced motility by decreasing epidermal growth factor receptor-mediated calpain activity. The Journal of Cell Biology, 146(1), 243–254. https://doi.org/10.1083/jcb.146.1.243.
Strieter, R. M., Polverini, P. J., Arenberg, D. A., Walz, A., Opdenakker, G., Van Damme, J., et al. (1995). Role of C-X-C chemokines as regulators of angiogenesis in lung cancer. Journal of Leukocyte Biology, 57(5), 752–762. https://doi.org/10.1002/jlb.57.5.752.
Strieter, R. M., Polverini, P. J., Kunkel, S. L., Arenberg, D. A., Burdick, M. D., Kasper, J., et al. (1995). The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. The Journal of Biological Chemistry, 270(45), 27348–27357. https://doi.org/10.1074/jbc.270.45.27348.
Dewald, O., Ren, G., Duerr, G. D., Zoerlein, M., Klemm, C., Gersch, C., et al. (2004). Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. The American Journal of Pathology, 164(2), 665–677. https://doi.org/10.1016/S0002-9440(10)63154-9.
Loetscher, M., Gerber, B., Loetscher, P., Jones, S. A., Piali, L., Clark-Lewis, I., et al. (1996). Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. The Journal of Experimental Medicine, 184(3), 963–969. https://doi.org/10.1084/jem.184.3.963.
Saxena, A., Bujak, M., Frunza, O., Dobaczewski, M., Gonzalez-Quesada, C., Lu, B., et al. (2014). CXCR3-independent actions of the CXC chemokine CXCL10 in the infarcted myocardium and in isolated cardiac fibroblasts are mediated through proteoglycans. Cardiovascular Research, 103(2), 217–227. https://doi.org/10.1093/cvr/cvu138.
Nagasawa, T., Hirota, S., Tachibana, K., Takakura, N., Nishikawa, S., Kitamura, Y., et al. (1996). Defects of B cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature, 382(6592), 635–638. https://doi.org/10.1038/382635a0.
Salvucci, O., Yao, L., Villalba, S., Sajewicz, A., Pittaluga, S., & Tosato, G. (2002). Regulation of endothelial cell branching morphogenesis by endogenous chemokine stromal-derived factor-1. Blood, 99(8), 2703–2711. https://doi.org/10.1182/blood.v99.8.2703.
Salcedo, R., Wasserman, K., Young, H. A., Grimm, M. C., Howard, O. M., Anver, M. R., et al. (1999). Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells: In vivo neovascularization induced by stromal-derived factor-1alpha. The American Journal of Pathology, 154(4), 1125–1135. https://doi.org/10.1016/s0002-9440(10)65365-5.
Pillarisetti, K., & Gupta, S. K. (2001). Cloning and relative expression analysis of rat stromal cell derived factor-1 (SDF-1)1: SDF-1 alpha mRNA is selectively induced in rat model of myocardial infarction. Inflammation, 25(5), 293–300. https://doi.org/10.1023/a:1012808525370.
Askari, A. T., Unzek, S., Popovic, Z. B., Goldman, C. K., Forudi, F., Kiedrowski, M., et al. (2003). Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet, 362(9385), 697–703. https://doi.org/10.1016/S0140-6736(03)14232-8.
Frangogiannis, N. G. (2011). The stromal cell-derived factor-1/CXCR4 axis in cardiac injury and repair. Journal of the American College of Cardiology, 58(23), 2424–2426. https://doi.org/10.1016/j.jacc.2011.08.031.
Uematsu, M., Yoshizaki, T., Shimizu, T., Obata, J. E., Nakamura, T., Fujioka, D., et al. (2015). Sustained myocardial production of stromal cell-derived factor-1alpha was associated with left ventricular adverse remodeling in patients with myocardial infarction. American Journal of Physiology. Heart and Circulatory Physiology, 309(10), H1764–H1771. https://doi.org/10.1152/ajpheart.00493.2015.
Ceradini, D. J., Kulkarni, A. R., Callaghan, M. J., Tepper, O. M., Bastidas, N., Kleinman, M. E., et al. (2004). Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nature Medicine, 10(8), 858–864. https://doi.org/10.1038/nm1075.
Abbott, J. D., Huang, Y., Liu, D., Hickey, R., Krause, D. S., & Giordano, F. J. (2004). Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation, 110(21), 3300–3305. https://doi.org/10.1161/01.CIR.0000147780.30124.CF.
Ma, J., Ge, J., Zhang, S., Sun, A., Shen, J., Chen, L., et al. (2005). Time course of myocardial stromal cell-derived factor 1 expression and beneficial effects of intravenously administered bone marrow stem cells in rats with experimental myocardial infarction. Basic Research in Cardiology, 100(3), 217–223. https://doi.org/10.1007/s00395-005-0521-z.
Saxena, A., Fish, J. E., White, M. D., Yu, S., Smyth, J. W., Shaw, R. M., et al. (2008). Stromal cell-derived factor-1alpha is cardioprotective after myocardial infarction. Circulation, 117(17), 2224–2231. https://doi.org/10.1161/CIRCULATIONAHA.107.694992.
Zhang, M., Mal, N., Kiedrowski, M., Chacko, M., Askari, A. T., Popovic, Z. B., et al. (2007). SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. The FASEB Journal, 21(12), 3197–3207. https://doi.org/10.1096/fj.06-6558com.
Sasaki, T., Fukazawa, R., Ogawa, S., Kanno, S., Nitta, T., Ochi, M., et al. (2007). Stromal cell-derived factor-1alpha improves infarcted heart function through angiogenesis in mice. Pediatrics International, 49(6), 966–971. https://doi.org/10.1111/j.1442-200X.2007.02491.x.
MacArthur Jr., J. W., Purcell, B. P., Shudo, Y., Cohen, J. E., Fairman, A., Trubelja, A., et al. (2013). Sustained release of engineered stromal cell-derived factor 1-alpha from injectable hydrogels effectively recruits endothelial progenitor cells and preserves ventricular function after myocardial infarction. Circulation, 128(11 Suppl 1), S79–S86. https://doi.org/10.1161/CIRCULATIONAHA.112.000343.
Macarthur Jr., J. W., Cohen, J. E., McGarvey, J. R., Shudo, Y., Patel, J. B., Trubelja, A., et al. (2014). Preclinical evaluation of the engineered stem cell chemokine stromal cell-derived factor 1alpha analog in a translational ovine myocardial infarction model. Circulation Research, 114(4), 650–659. https://doi.org/10.1161/CIRCRESAHA.114.302884.
Elmadbouh, I., Haider, H., Jiang, S., Idris, N. M., Lu, G., & Ashraf, M. (2007). Ex vivo delivered stromal cell-derived factor-1alpha promotes stem cell homing and induces angiomyogenesis in the infarcted myocardium. Journal of Molecular and Cellular Cardiology, 42(4), 792–803. https://doi.org/10.1016/j.yjmcc.2007.02.001.
Mayorga, M. E., Kiedrowski, M., McCallinhart, P., Forudi, F., Ockunzzi, J., Weber, K., et al. (2018). Role of SDF-1:CXCR4 in impaired post-myocardial infarction cardiac repair in diabetes. Stem Cells Translational Medicine, 7(1), 115–124. https://doi.org/10.1002/sctm.17-0172.
Cheng, M., Huang, K., Zhou, J., Yan, D., Tang, Y. L., Zhao, T. C., et al. (2015). A critical role of Src family kinase in SDF-1/CXCR4-mediated bone-marrow progenitor cell recruitment to the ischemic heart. Journal of Molecular and Cellular Cardiology, 81, 49–53. https://doi.org/10.1016/j.yjmcc.2015.01.024.
Wang, M., Hu, R., Yang, Y., Xiang, L., & Mu, Y. (2019). In vivo ultrasound molecular imaging of SDF-1 expression in a swine model of acute myocardial infarction. Frontiers in Pharmacology, 10, 899. https://doi.org/10.3389/fphar.2019.00899.
Huang, F. Y., Xia, T. L., Li, J. L., Li, C. M., Zhao, Z. G., Lei, W. H., et al. (2019). The bifunctional SDF-1-AnxA5 fusion protein protects cardiac function after myocardial infarction. Journal of Cellular and Molecular Medicine, 23(11), 7673–7684. https://doi.org/10.1111/jcmm.14640.
Goldstone, A. B., Burnett, C. E., Cohen, J. E., Paulsen, M. J., Eskandari, A., Edwards, B. E., et al. (2018). SDF 1-alpha attenuates myocardial injury without altering the direct contribution of circulating cells. Journal of Cardiovascular Translational Research, 11(4), 274–284. https://doi.org/10.1007/s12265-017-9772-y.
Liu, Y., Gao, S., Wang, Z., Yang, Y., Huo, H., & Tian, X. (2016). Effect of stromal cell-derived factor-1 on myocardial apoptosis and cardiac function recovery in rats with acute myocardial infarction. Experimental and Therapeutic Medicine, 12(5), 3282–3286. https://doi.org/10.3892/etm.2016.3770.
Hu, X., Dai, S., Wu, W. J., Tan, W., Zhu, X., Mu, J., et al. (2007). Stromal cell derived factor-1 alpha confers protection against myocardial ischemia/reperfusion injury: role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis. Circulation, 116(6), 654–663. https://doi.org/10.1161/CIRCULATIONAHA.106.672451.
Chen, J., Chemaly, E., Liang, L., Kho, C., Lee, A., Park, J., et al. (2010). Effects of CXCR4 gene transfer on cardiac function after ischemia-reperfusion injury. The American Journal of Pathology, 176(4), 1705–1715. https://doi.org/10.2353/ajpath.2010.090451.
Dai, S., Yuan, F., Mu, J., Li, C., Chen, N., Guo, S., et al. (2010). Chronic AMD3100 antagonism of SDF-1alpha-CXCR4 exacerbates cardiac dysfunction and remodeling after myocardial infarction. Journal of Molecular and Cellular Cardiology, 49(4), 587–597. https://doi.org/10.1016/j.yjmcc.2010.07.010.
Proulx, C., El-Helou, V., Gosselin, H., Clement, R., Gillis, M. A., Villeneuve, L., et al. (2007). Antagonism of stromal cell-derived factor-1alpha reduces infarct size and improves ventricular function after myocardial infarction. Pflügers Archiv, 455(2), 241–250. https://doi.org/10.1007/s00424-007-0284-5.
Jujo, K., Hamada, H., Iwakura, A., Thorne, T., Sekiguchi, H., Clarke, T., et al. (2010). CXCR4 blockade augments bone marrow progenitor cell recruitment to the neovasculature and reduces mortality after myocardial infarction. Proceedings of the National Academy of Sciences of the United States of America, 107(24), 11008–11013. https://doi.org/10.1073/pnas.0914248107.
Hao, H., Hu, S., Chen, H., Bu, D., Zhu, L., Xu, C., et al. (2017). Loss of endothelial CXCR7 impairs vascular homeostasis and cardiac remodeling after myocardial infarction: implications for cardiovascular drug discovery. Circulation, 135(13), 1253–1264. https://doi.org/10.1161/CIRCULATIONAHA.116.023027.
Das, S., Goldstone, A. B., Wang, H., Farry, J., D'Amato, G., Paulsen, M. J., et al. (2019). A unique collateral artery development program promotes neonatal heart regeneration. Cell, 176(5), 1128-1142 e1118, doi:https://doi.org/10.1016/j.cell.2018.12.023.
Dewald, O., Zymek, P., Winkelmann, K., Koerting, A., Ren, G., Abou-Khamis, T., et al. (2005). CCL2/Monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circulation Research, 96(8), 881–889. https://doi.org/10.1161/01.RES.0000163017.13772.3a.
Ono, K., Matsumori, A., Furukawa, Y., Igata, H., Shioi, T., Matsushima, K., et al. (1999). Prevention of myocardial reperfusion injury in rats by an antibody against monocyte chemotactic and activating factor/monocyte chemoattractant protein-1. Laboratory Investigation, 79(2), 195–203.
Tarzami, S. T., Cheng, R., Miao, W., Kitsis, R. N., & Berman, J. W. (2002). Chemokine expression in myocardial ischemia: MIP-2 dependent MCP-1 expression protects cardiomyocytes from cell death. Journal of Molecular and Cellular Cardiology, 34(2), 209–221. https://doi.org/10.1006/jmcc.2001.1503.
Pinto, A. R., Ilinykh, A., Ivey, M. J., Kuwabara, J. T., D’Antoni, M. L., Debuque, R., et al. (2016). Revisiting cardiac cellular composition. Circulation Research, 118(3), 400–409. https://doi.org/10.1161/CIRCRESAHA.115.307778.
Epelman, S., Lavine, K. J., Beaudin, A. E., Sojka, D. K., Carrero, J. A., Calderon, B., et al. (2014). Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity, 40(1), 91–104. https://doi.org/10.1016/j.immuni.2013.11.019.
Epelman, S., Lavine, K. J., & Randolph, G. J. (2014). Origin and functions of tissue macrophages. Immunity, 41(1), 21–35. https://doi.org/10.1016/j.immuni.2014.06.013.
Lavine, K. J., Epelman, S., Uchida, K., Weber, K. J., Nichols, C. G., Schilling, J. D., et al. (2014). Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proceedings of the National Academy of Sciences of the United States of America, 111(45), 16029–16034. https://doi.org/10.1073/pnas.1406508111.
Leid, J., Carrelha, J., Boukarabila, H., Epelman, S., Jacobsen, S. E., & Lavine, K. J. (2016). Primitive embryonic macrophages are required for coronary development and maturation. Circulation Research, 118(10), 1498–1511. https://doi.org/10.1161/CIRCRESAHA.115.308270.
Bajpai, G., Bredemeyer, A., Li, W., Zaitsev, K., Koenig, A. L., Lokshina, I., et al. (2019). Tissue resident CCR2- and CCR2+ cardiac macrophages differentially orchestrate monocyte recruitment and fate specification following myocardial injury. Circulation Research, 124(2), 263–278. https://doi.org/10.1161/CIRCRESAHA.118.314028.
Heidt, T., Courties, G., Dutta, P., Sager, H. B., Sebas, M., Iwamoto, Y., et al. (2014). Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circulation Research, 115(2), 284–295. https://doi.org/10.1161/CIRCRESAHA.115.303567.
Frangogiannis, N. G., Mendoza, L. H., Ren, G., Akrivakis, S., Jackson, P. L., Michael, L. H., et al. (2003). MCSF expression is induced in healing myocardial infarcts and may regulate monocyte and endothelial cell phenotype. American Journal of Physiology. Heart and Circulatory Physiology, 285(2), H483–H492.
Sager, H. B., Hulsmans, M., Lavine, K. J., Moreira, M. B., Heidt, T., Courties, G., et al. (2016). Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circulation Research, 119(7), 853–864. https://doi.org/10.1161/CIRCRESAHA.116.309001.
Liehn, E. A., Piccinini, A. M., Koenen, R. R., Soehnlein, O., Adage, T., Fatu, R., et al. (2010). A new monocyte chemotactic protein-1/chemokine CC motif ligand-2 competitor limiting neointima formation and myocardial ischemia/reperfusion injury in mice. Journal of the American College of Cardiology, 56(22), 1847–1857. https://doi.org/10.1016/j.jacc.2010.04.066.
Al-Amran, F. G., Manson, M. Z., Hanley, T. K., & Hainz, D. L. (2014). Blockade of the monocyte chemoattractant protein-1 receptor pathway ameliorates myocardial injury in animal models of ischemia and reperfusion. Pharmacology, 93(5-6), 296–302. https://doi.org/10.1159/000363657.
Grisanti, L. A., Traynham, C. J., Repas, A. A., Gao, E., Koch, W. J., & Tilley, D. G. (2016). beta2-Adrenergic receptor-dependent chemokine receptor 2 expression regulates leukocyte recruitment to the heart following acute injury. Proceedings of the National Academy of Sciences of the United States of America, 113(52), 15126–15131. https://doi.org/10.1073/pnas.1611023114.
Wang, J., Seo, M. J., Deci, M. B., Weil, B. R., Canty, J. M., & Nguyen, J. (2018). Effect of CCR2 inhibitor-loaded lipid micelles on inflammatory cell migration and cardiac function after myocardial infarction. International Journal of Nanomedicine, 13, 6441–6451. https://doi.org/10.2147/IJN.S178650.
Leuschner, F., Dutta, P., Gorbatov, R., Novobrantseva, T. I., Donahoe, J. S., Courties, G., et al. (2011). Therapeutic siRNA silencing in inflammatory monocytes in mice. Nature Biotechnology, 29(11), 1005–1010. https://doi.org/10.1038/nbt.1989.
Lu, W., Xie, Z., Tang, Y., Bai, L., Yao, Y., Fu, C., et al. (2015). Photoluminescent mesoporous silicon nanoparticles with siCCR2 improve the effects of mesenchymal stromal cell transplantation after acute myocardial infarction. Theranostics, 5(10), 1068–1082. https://doi.org/10.7150/thno.11517.
Kaikita, K., Hayasaki, T., Okuma, T., Kuziel, W. A., Ogawa, H., & Takeya, M. (2004). Targeted deletion of CC chemokine receptor 2 attenuates left ventricular remodeling after experimental myocardial infarction. The American Journal of Pathology, 165(2), 439–447. https://doi.org/10.1016/S0002-9440(10)63309-3.
Nahrendorf, M., Swirski, F. K., Aikawa, E., Stangenberg, L., Wurdinger, T., Figueiredo, J. L., et al. (2007). The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. The Journal of Experimental Medicine, 204(12), 3037–3047. https://doi.org/10.1084/jem.20070885.
Gavrilin, M. A., Deucher, M. F., Boeckman, F., & Kolattukudy, P. E. (2000). Monocyte chemotactic protein 1 upregulates IL-1beta expression in human monocytes. Biochemical and Biophysical Research Communications, 277(1), 37–42. https://doi.org/10.1006/bbrc.2000.3619.
Jiang, Y., Beller, D. I., Frendl, G., & Graves, D. T. (1992). Monocyte chemoattractant protein-1 regulates adhesion molecule expression and cytokine production in human monocytes. Journal of Immunology, 148(8), 2423–2428.
Sierra-Filardi, E., Nieto, C., Dominguez-Soto, A., Barroso, R., Sanchez-Mateos, P., Puig-Kroger, A., et al. (2014). CCL2 shapes macrophage polarization by GM-CSF and M-CSF: identification of CCL2/CCR2-dependent gene expression profile. Journal of Immunology, 192(8), 3858–3867. https://doi.org/10.4049/jimmunol.1302821.
Frangogiannis, N. G., Dewald, O., Xia, Y., Ren, G., Haudek, S., Leucker, T., et al. (2007). Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation, 115(5), 584–592.
Schenk, S., Mal, N., Finan, A., Zhang, M., Kiedrowski, M., Popovic, Z., et al. (2007). Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells, 25(1), 245–251. https://doi.org/10.1634/stemcells.2006-0293.
Montecucco, F., Braunersreuther, V., Lenglet, S., Delattre, B. M., Pelli, G., Buatois, V., et al. (2012). CC chemokine CCL5 plays a central role impacting infarct size and post-infarction heart failure in mice. European Heart Journal, 33(15), 1964–1974. https://doi.org/10.1093/eurheartj/ehr127.
Huang, Y., Wang, D., Wang, X., Zhang, Y., Liu, T., Chen, Y., et al. (2016). Abrogation of CC chemokine receptor 9 ameliorates ventricular remodeling in mice after myocardial infarction. Scientific Reports, 6, 32660. https://doi.org/10.1038/srep32660.
Jiang, Y., Bai, J., Tang, L., Zhang, P., & Pu, J. (2015). Anti-CCL21 antibody attenuates infarct size and improves cardiac remodeling after myocardial infarction. Cellular Physiology and Biochemistry, 37(3), 979–990. https://doi.org/10.1159/000430224.
Parissis, J. T., Adamopoulos, S., Venetsanou, K. F., Mentzikof, D. G., Karas, S. M., & Kremastinos, D. T. (2002). Serum profiles of C-C chemokines in acute myocardial infarction: possible implication in postinfarction left ventricular remodeling. Journal of Interferon & Cytokine Research, 22(2), 223–229. https://doi.org/10.1089/107999002753536194.
Braunersreuther, V., Montecucco, F., Pelli, G., Galan, K., Proudfoot, A. E., Belin, A., et al. (2013). Treatment with the CC chemokine-binding protein Evasin-4 improves post-infarction myocardial injury and survival in mice. Thrombosis and Haemostasis, 110(4), 807–825. https://doi.org/10.1160/TH13-04-0297.
Liehn, E. A., Merx, M. W., Postea, O., Becher, S., Djalali-Talab, Y., Shagdarsuren, E., et al. (2008). Ccr1 deficiency reduces inflammatory remodelling and preserves left ventricular function after myocardial infarction. Journal of Cellular and Molecular Medicine, 12(2), 496–506. https://doi.org/10.1111/j.1582-4934.2007.00194.x.
Combadiere, C., Ahuja, S. K., Tiffany, H. L., & Murphy, P. M. (1996). Cloning and functional expression of CC CKR5, a human monocyte CC chemokine receptor selective for MIP-1(alpha), MIP-1(beta), and RANTES. Journal of Leukocyte Biology, 60(1), 147–152. https://doi.org/10.1002/jlb.60.1.147.
Dobaczewski, M., Xia, Y., Bujak, M., Gonzalez-Quesada, C., & Frangogiannis, N. G. (2010). CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. The American Journal of Pathology, 176(5), 2177–2187. https://doi.org/10.2353/ajpath.2010.090759.
Zamilpa, R., Kanakia, R., Cigarroa, J. t., Dai, Q., Escobar, G. P., Martinez, H., et al. (2011). CC chemokine receptor 5 deletion impairs macrophage activation and induces adverse remodeling following myocardial infarction. American Journal of Physiology. Heart and Circulatory Physiology, 300(4), H1418–H1426. https://doi.org/10.1152/ajpheart.01002.2010.
Xuan, W., Liao, Y., Chen, B., Huang, Q., Xu, D., Liu, Y., et al. (2011). Detrimental effect of fractalkine on myocardial ischaemia and heart failure. Cardiovascular Research, 92(3), 385–393. https://doi.org/10.1093/cvr/cvr221.
Husberg, C., Nygard, S., Finsen, A. V., Damas, J. K., Frigessi, A., Oie, E., et al. (2008). Cytokine expression profiling of the myocardium reveals a role for CX3CL1 (fractalkine) in heart failure. Journal of Molecular and Cellular Cardiology, 45(2), 261–269.
Boag, S. E., Das, R., Shmeleva, E. V., Bagnall, A., Egred, M., Howard, N., et al. (2015). T lymphocytes and fractalkine contribute to myocardial ischemia/reperfusion injury in patients. The Journal of Clinical Investigation, 125(8), 3063–3076. https://doi.org/10.1172/JCI80055.
Xu, B., Qian, Y., Zhao, Y., Fang, Z., Tang, K., Zhou, N., et al. (2019). Prognostic value of fractalkine/CX3CL1 concentration in patients with acute myocardial infarction treated with primary percutaneous coronary intervention. Cytokine, 113, 365–370. https://doi.org/10.1016/j.cyto.2018.10.006.
Gu, X., Xu, J., Yang, X. P., Peterson, E., & Harding, P. (2015). Fractalkine neutralization improves cardiac function after myocardial infarction. Experimental Physiology, 100(7), 805–817. https://doi.org/10.1113/EP085104.
Draganova, L., Redgrave, R., Tual-Chalot, S., Marsh, S., Arthur, H., & Spyridopoulos, I. (2019). BS31 The role of fractalkine and CX3CR1-expressing lymphocytes during myocardial ischaemia/reperfusion injury. Heart, 105(Suppl 6), A160–A160. https://doi.org/10.1136/heartjnl-2019-BCS.194.
Huang, S., & Frangogiannis, N. G. (2018). Anti-inflammatory therapies in myocardial infarction: failures, hopes and challenges. British Journal of Pharmacology, 175(9), 1377–1400. https://doi.org/10.1111/bph.14155.
Chung, E. S., Miller, L., Patel, A. N., Anderson, R. D., Mendelsohn, F. O., Traverse, J., et al. (2015). Changes in ventricular remodelling and clinical status during the year following a single administration of stromal cell-derived factor-1 non-viral gene therapy in chronic ischaemic heart failure patients: the STOP-HF randomized phase II trial. European Heart Journal, 36(33), 2228–2238. https://doi.org/10.1093/eurheartj/ehv254.
Agarwal, U., Ghalayini, W., Dong, F., Weber, K., Zou, Y. R., Rabbany, S. Y., et al. (2010). Role of cardiac myocyte CXCR4 expression in development and left ventricular remodeling after acute myocardial infarction. Circulation Research, 107(5), 667–676. https://doi.org/10.1161/CIRCRESAHA.110.223289.
Liehn, E. A., Tuchscheerer, N., Kanzler, I., Drechsler, M., Fraemohs, L., Schuh, A., et al. (2011). Double-edged role of the CXCL12/CXCR4 axis in experimental myocardial infarction. Journal of the American College of Cardiology, 58(23), 2415–2423. https://doi.org/10.1016/j.jacc.2011.08.033.
Hayasaki, T., Kaikita, K., Okuma, T., Yamamoto, E., Kuziel, W. A., Ogawa, H., et al. (2006). CC chemokine receptor-2 deficiency attenuates oxidative stress and infarct size caused by myocardial ischemia-reperfusion in mice. Circulation Journal, 70(3), 342–351. https://doi.org/10.1253/circj.70.342.
Hayashidani, S., Tsutsui, H., Shiomi, T., Ikeuchi, M., Matsusaka, H., Suematsu, N., et al. (2003). Anti-monocyte chemoattractant protein-1 gene therapy attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation, 108(17), 2134–2140. https://doi.org/10.1161/01.CIR.0000092890.29552.22.
Morimoto, H., Hirose, M., Takahashi, M., Kawaguchi, M., Ise, H., Kolattukudy, P. E., et al. (2008). MCP-1 induces cardioprotection against ischaemia/reperfusion injury: role of reactive oxygen species. Cardiovascular Research, 78(3), 554–562. https://doi.org/10.1093/cvr/cvn035.
Shen, B., Li, J., Gao, L., Zhang, J., & Yang, B. (2013). Role of CC-chemokine receptor 5 on myocardial ischemia-reperfusion injury in rats. Molecular and Cellular Biochemistry, 378(1-2), 137–144. https://doi.org/10.1007/s11010-013-1604-z.
Funding
Dr Frangogiannis’ laboratory is supported by NIH R01 grants HL76246, HL85440, and HL149407, and by Department of Defense grants PR151029 and PR181464. Dr Chen is supported by an American Heart Association post-doctoral award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Associate Editor Saskia de Jager oversaw the review of this article
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, B., Frangogiannis, N.G. Chemokines in Myocardial Infarction. J. of Cardiovasc. Trans. Res. 14, 35–52 (2021). https://doi.org/10.1007/s12265-020-10006-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-020-10006-7