Abstract
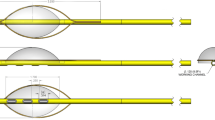
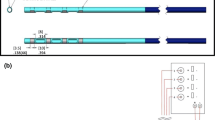
Endomyocardial delivery in the setting of active left ventricular assist device (LVAD) support has rarely been studied. The objective was to establish a protocol for endomyocardial injections during LVAD support without compromising mechanical circulation. LVAD implantation was performed in four pigs. A curved needle catheter was percutaneously inserted into the right carotid artery and positioned into the left ventricle under fluoroscopic guidance. In the setting of increasing LVAD flows (2.3–3.1 l/min), percutaneous methylene blue dye administration into the myocardium proceeded without complications in all pigs. Transection of excised hearts revealed an anterior, lateral, inferior, and septal wall distribution of methylene blue documenting injections in all four regions of the left ventricle. Ex vivo, the catheter could be maneuvered close to the LVAD inflow cannula despite augmentation of LVAD flow up to 5 l/min. Endomyocardial injections during LVAD support was found to be feasible and safe with the curved needle catheter.

Similar content being viewed by others
Abbreviations
- LVAD:
-
Left ventricular assist device
References
Mosterd, A., & Hoes, A. W. (2007). Clinical epidemiology of heart failure. Heart, 93, 1137–1146.
Redfield, M. M., Jacobsen, S. J., Burnett, J. C., Jr., et al. (2003). Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA, 289, 194–202.
Slaughter, M. S., Rogers, J. G., Milano, C. A., Russel, S. D., Conte, J. V., Feldman, D., et al. (2009). Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl Med, 361, 2241–2251.
Birks, E. J. (2013). Molecular changes after left ventricular assist device support for heart failure. Circulation Research, 113, 777–791.
Hall, J. L., Fermin, D. R., Birks, E. J., Barton, P. J., Slaughter, M., & Eckman, P. (2011). Clinical, molecular, and genomic changes in response to a left ventricular assist device. Journal of the American College of Cardiology, 57, 641–652.
Behfar, A., Crespo-Diaz, R., Terzic, A., & Gersh, B. J. (2014). Cell therapy for cardiac repair—lessons from clinical trials. Nature Reviews Cardiology, 11, 232–246.
Ibrahim, M., & Yacoub, M. H. (2014). Bridge to recovery and weaning protocols. Heart Failure Clinics, 10, S47–S55.
Anastasiadis, K., Antonitsis, P., Argiriadou, H., Koliakos, G., Doumas, A., Khayat, A., et al. (2011). Hybrid approach of ventricular assist device and autologous bone marrow stem cells implantation in end-stage ischemic heart failure enhances myocardial reperfusion. Journal of Translational Medicine, 9, 12.
Ascheim, D. D., Gelijns, A. C., Goldstein, D., Moye, L. A., Smedira, N., Lee, S., et al. (2014). Mesenchymal precursor cells as adjunctive therapy in recipients of contemporary left ventricular assist devices. Circulation, 129, 2287–2296.
Zheng, Y., Sampaio, L. C., Li, K., Silva, G. V., Cabreira-Hansen, M., Vela, D., et al. (2013). Safety and feasibility of mapping and stem cell delivery in the presence of an implanted left ventricular assist device: a preclinical investigation in sheep. Texas Heart Institute Journal, 40, 229–234.
Behfar, A., Latere, J. P., Bartunek, J., Homsy, C., Daro, D., Crespo-Diaz, R. J., et al. (2013). Optimized delivery system achieves enhanced endomyocardial stem cell retention. Circulation. Cardiovascular Interventions, 6, 708–710.
Vikholm, P., Schiller, P., Johansson, J., & Hellgren, L. (2014). Cavoaortic shunt improves hemodynamics with preserved oxygen delivery in experimental right ventricular failure during left ventricular assist device therapy. Journal of Thoracic and Cardiovascular Surgery, 147, 625–631.
Nakamuta, J. S., Danoviz, M. E., Marques, F. L., dos Santos, L., Becker, C., Gonçalves, G. A., et al. (2009). Cell therapy attenuates cardiac dysfunction post myocardial infarction: effect of timing, routes of injection and a fibrin scaffold. PLoS One, 4, e6005.
Telukuntla, K. S., Suncion, V. Y., Schulman, I. H., & Hare, J. M. (2013). The advancing field of cell-based therapy: insights and lessons from clinical trials. J Am Heart Assoc, 2, e000338.
Acknowledgments
We thank Andreas Rück, MD, PhD at the Department of Cardiology at Karolinska University Hospital for the technical support and Tor Kristoffersen, Innova Medical AS, for providing the HeartWare LVAD system for experimental use.
Sources of Funding
This study was supported in part by grants from the Karolinska Institutet, Stockholm County Council Strategic Grants, the Swedish Heart-Lung Foundation, the Mats Kleberg Foundation, Mayo Clinic Center for Regenerative Medicine, Marriott Foundation, Michael S. and Mary Sue Shannon Family, and Russ and Kathy VanCleve Foundation.
Conflict of Interest
Aymeric Seron and Warren Sherman are affiliated with Celyad S.A.
Statement on the Welfare of Animals
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the Uppsala Ethical Committee of Animal Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Lorrie Kirshenbaum oversaw the review of this article
Electronic supplementary material
Below is the link to the electronic supplementary material.
Animation of the curved needle catheter. (MP4 38816 kb)
Methylene blue dye administered percutaneously into the endocardium and myocardium using the curved needle catheter during step-wise increased LVAD flow and fluoroscopic guidance. (M4V 9087 kb)
In vitro study with the LVAD run in buffered glucose solution carrier. The catheter is easily maneuvered close to the LVAD inflow cannula during a LVAD estimated flow of 5 L/min. (MOV 19975 kb)
Rights and permissions
About this article
Cite this article
Dalén, M., Behfar, A., Terzic, A. et al. Percutaneous Fluoroscopic-Guided Endomyocardial Delivery in an Experimental Model of Left Ventricular Assist Device Support. J. of Cardiovasc. Trans. Res. 8, 381–384 (2015). https://doi.org/10.1007/s12265-015-9642-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-015-9642-4