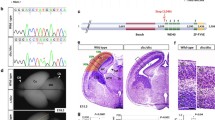
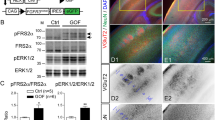
Abstract
Heterozygous loss-of-function variants of FOXP4 are associated with neurodevelopmental disorders (NDDs) that exhibit delayed speech development, intellectual disability, and congenital abnormalities. The etiology of NDDs is unclear. Here we found that FOXP4 and N-cadherin are expressed in the nuclei and apical end-feet of radial glial cells (RGCs), respectively, in the mouse neocortex during early gestation. Knockdown or dominant-negative inhibition of Foxp4 abolishes the apical condensation of N-cadherin in RGCs and the integrity of neuroepithelium in the ventricular zone (VZ). Inhibition of Foxp4 leads to impeded radial migration of cortical neurons and ectopic neurogenesis from the proliferating VZ. The ectopic differentiation and deficient migration disappear when N-cadherin is over-expressed in RGCs. The data indicate that Foxp4 is essential for N-cadherin-based adherens junctions, the loss of which leads to periventricular heterotopias. We hypothesize that FOXP4 variant-associated NDDs may be caused by disruption of the adherens junctions and malformation of the cerebral cortex.








Similar content being viewed by others
References
Teufel A, Wong EA, Mukhopadhyay M, Malik N, Westphal H. FoxP4, a novel forkhead transcription factor. Biochim Biophys Acta 2003, 1627: 147–152.
Bowers JM, Konopka G. The role of the FOXP family of transcription factors in ASD. Dis Markers 2012, 33: 251–260.
Lai CSL, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature 2001, 413: 519–523.
Morgan AT, Webster R. Aetiology of childhood apraxia of speech: A clinical practice update for paediatricians. J Paediatr Child Health 2018, 54: 1090–1095.
Hamdan FF, Daoud H, Rochefort D, Piton A, Gauthier J, Langlois M. De novo mutations in FOXP1 in cases with intellectual disability, autism, and language impairment. Am J Hum Genet 2010, 87: 671–678.
O’Roak BJ, Deriziotis P, Lee C, Vives L, Schwartz JJ, Girirajan S, et al. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat Genet 2011, 43: 585–589.
Sollis E, Graham SA, Vino A, Froehlich H, Vreeburg M, Dimitropoulou D, et al. Identification and functional characterization of de novo FOXP1 variants provides novel insights into the etiology of neurodevelopmental disorder. Hum Mol Genet 2015, 25: 546–557.
Medvedeva VP, Rieger MA, Vieth B, Mombereau C, Ziegenhain C, Ghosh T, et al. Altered social behavior in mice carrying a cortical Foxp2 deletion. Hum Mol Genet 2018, 28: 701–717.
Bacon C, Schneider M, Le Magueresse C, Froehlich H, Sticht C, Gluch C, et al. Brain-specific Foxp1 deletion impairs neuronal development and causes autistic-like behaviour. Mol Psychiatry 2015, 20: 632–639.
Snijders Blok L, Vino A, den Hoed J, Underhill HR, Monteil D, Li H, et al. Heterozygous variants that disturb the transcriptional repressor activity of FOXP4 cause a developmental disorder with speech/language delays and multiple congenital abnormalities. Genet Med 2021, 23: 534–542.
Noctor SC, Martínez-Cerdeño V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci 2004, 7: 136–144.
Nadarajah B, Parnavelas JG. Modes of neuronal migration in the developing cerebral cortex. Nat Rev Neurosci 2002, 3: 423–432.
Kwan KY, Sestan N, Anton ES. Transcriptional co-regulation of neuronal migration and laminar identity in the neocortex. Development 2012, 139: 1535–1546.
Li X, Xiao J, Fröhlich H, Tu X, Li L, Xu Y, et al. Foxp1 regulates cortical radial migration and neuronal morphogenesis in developing cerebral cortex. PLoS One 2015, 10: e0127671.
Pearson CA, Moore DM, Tucker HO, Dekker JD, Hu H, Miquelajauregui A, et al. Foxp1 regulates neural stem cell self-renewal and bias toward deep layer cortical fates. Cell Rep 2020, 30: 1964-1981.e3.
Tsui D, Vessey JP, Tomita H, Kaplan DR, Miller FD. FoxP2 regulates neurogenesis during embryonic cortical development. J Neurosci 2013, 33: 244–258.
Garcia-Calero E, Botella-Lopez A, Bahamonde O, Perez-Balaguer A, Martinez S. FoxP2 protein levels regulate cell morphology changes and migration patterns in the vertebrate developing telencephalon. Brain Struct Funct 2016, 221: 2905–2917.
Buchsbaum IY, Cappello S. Neuronal migration in the CNS during development and disease: Insights from in vivo and in vitro models. Development 2019, 146: dev163766.
Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 2009, 32: 149–184.
Shikanai M, Nakajima K, Kawauchi T. N-Cadherin regulates radial glial fiber-dependent migration of cortical locomoting neurons. Commun Integr Biol 2011, 4: 326–330.
Meng W, Takeichi M. Adherens junction: Molecular architecture and regulation. Cold Spring Harb Perspect Biol 2009, 1: a002899.
Miyamoto Y, Sakane F, Hashimoto K. N-cadherin-based adherens junction regulates the maintenance, proliferation, and differentiation of neural progenitor cells during development. Cell Adhesion Migr 2015, 9: 183–192.
Veeraval L, O’Leary CJ, Cooper HM. Adherens junctions: Guardians of cortical development. Front Cell Dev Biol 2020, 8: 6.
Zhang J, Woodhead GJ, Swaminathan SK, Noles SR, McQuinn ER, Pisarek AJ, et al. Cortical neural precursors inhibit their own differentiation via N-cadherin maintenance of beta-catenin signaling. Dev Cell 2010, 18: 472–479.
Farkas LM. The cell biology of neural stem and progenitor cells and its significance for their proliferation versus differentiation during mammalian brain development. Curr Opin Cell Biol 2008, 20: 707–715.
Rousso DL, Pearson CA, Gaber ZB, Miquelajauregui A, Li S, Portera-Cailliau C, et al. Foxp-mediated suppression of N-cadherin regulates neuroepithelial character and progenitor maintenance in the CNS. Neuron 2012, 74: 314–330.
Sheen VL, Basel-Vanagaite L, Goodman JR, Scheffer IE, Bodell A, Ganesh VS, et al. Etiological heterogeneity of familial periventricular heterotopia and hydrocephalus. Brain Dev 2004, 26: 326–334.
Sheen VL, Ganesh VS, Topcu M, Sebire G, Bodell A, Hill RS, et al. Mutations in ARFGEF2 implicate vesicle trafficking in neural progenitor proliferation and migration in the human cerebral cortex. Nat Genet 2004, 36: 69–76.
Fox JW, Lamperti ED, Ekşioğlu YZ, Hong SE, Feng Y, Graham DA, et al. Mutations in filamin 1 prevent migration of cerebral cortical neurons in human periventricular heterotopia. Neuron 1998, 21: 1315–1325.
Chen JG, Rasin MR, Kwan KY, Sestan N. Zfp312 is required for subcortical axonal projections and dendritic morphology of deep-layer pyramidal neurons of the cerebral cortex. Proc Natl Acad Sci U S A 2005, 102: 17792–17797.
Takahashi K, Liu FC, Hirokawa K, Takahashi H. Expression of Foxp4 in the developing and adult rat forebrain. J Neurosci Res 2008, 86: 3106–3116.
Temple S. The development of neural stem cells. Nature 2001, 414: 112–117.
Sanghvi-Shah R, Weber GF. Intermediate filaments at the junction of mechanotransduction, migration, and development. Front Cell Dev Biol 2017, 5: 81.
Shao W, Yang J, He M, Yu XY, Lee CH, Yang Z, et al. Centrosome anchoring regulates progenitor properties and cortical formation. Nature 2020, 580: 106–112.
Gärtner A, Fornasiero EF, Munck S, Vennekens K, Seuntjens E, Huttner WB, et al. N-cadherin specifies first asymmetry in developing neurons. EMBO J 2012, 31: 1893–1903.
Kawauchi T, Sekine K, Shikanai M, Chihama K, Tomita K, Kubo KI, et al. Rab GTPases-dependent endocytic pathways regulate neuronal migration and maturation through N-cadherin trafficking. Neuron 2010, 67: 588–602.
Huber AH, Stewart DB, Laurents DV, Nelson WJ, Weis WI. The cadherin cytoplasmic domain is unstructured in the absence of beta-catenin. A possible mechanism for regulating cadherin turnover. J Biol Chem 2001, 276: 12301–12309.
Mutch CA, Schulte JD, Olson E, Chenn A. Beta-catenin signaling negatively regulates intermediate progenitor population numbers in the developing cortex. PLoS One 2010, 5: e12376.
Wang J, Li T, Wang JL, Xu Z, Meng W, Wu QF. Talpid3-mediated centrosome integrity restrains neural progenitor delamination to sustain neurogenesis by stabilizing adherens junctions. Cell Rep 2020, 33: 108495.
Romero DM, Poirier K, Belvindrah R, Moutkine I, Houllier A, LeMoing AG, et al. Novel role of the synaptic scaffold protein Dlgap4 in ventricular surface integrity and neuronal migration during cortical development. Nat Commun 2022, 13: 2746.
Wei C, Sun M, Sun X, Meng H, Li Q, Gao K, et al. RhoGEF trio regulates radial migration of projection neurons via its distinct domains. Neurosci Bull 2022, 38: 249–262.
Linford A, Yoshimura SI, Bastos RN, Langemeyer L, Gerondopoulos A, Rigden DJ, et al. Rab14 and its exchange factor FAM116 link endocytic recycling and adherens junction stability in migrating cells. Dev Cell 2012, 22: 952–966.
Tai CY, Mysore SP, Chiu C, Schuman EM. Activity-regulated N-cadherin endocytosis. Neuron 2007, 54: 771–785.
Wong KK, Gascoyne DM, Brown PJ, Soilleux EJ, Snell C, Chen H, et al. Reciprocal expression of the endocytic protein HIP1R and its repressor FOXP1 predicts outcome in R-CHOP-treated diffuse large B-cell lymphoma patients. Leukemia 2014, 28: 362–372.
Roura S, Miravet S, Piedra J, García de Herreros A, Duñach M. Regulation of E-cadherin/catenin association by tyrosine phosphorylation. J Biol Chem 1999, 274: 36734–36740.
Niu Y, Wang G, Li Y, Guo W, Guo Y, Dong Z. LncRNA FOXP4-AS1 promotes the progression of esophageal squamous cell carcinoma by interacting with MLL2/H3K4me3 to upregulate FOXP4. Front Oncol 2021, 11: 773864.
Christopher AC, Koshy K. Neurological picture. Periventricular heterotopia in refractory epilepsy. J Neurol Neurosurg Psychiatry 2013, 84: 1136–1137.
Acknowledgements
This work was supported by the Wenzhou Municipal Science and Technology Bureau (Y20210901), the Natural Science Foundation of Zhejiang Province (LQ20H090001), and the Scientific Research Fund of Wenling Science and Technology Bureau (2018C320001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing financial interests to declare.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Zou, S., Tu, X. et al. Inhibition of Foxp4 Disrupts Cadherin-based Adhesion of Radial Glial Cells, Leading to Abnormal Differentiation and Migration of Cortical Neurons in Mice. Neurosci. Bull. 39, 1131–1145 (2023). https://doi.org/10.1007/s12264-022-01004-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-022-01004-7