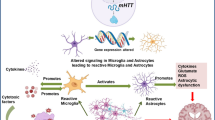
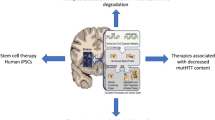
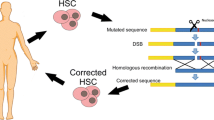
Abstract
Huntington’s disease (HD) is an autosomal dominantly-inherited neurodegenerative disease, which is caused by CAG trinucleotide expansion in exon 1 of the Huntingtin (HTT) gene. Although HD is a rare disease, its monogenic nature makes it an ideal model in which to understand pathogenic mechanisms and to develop therapeutic strategies for neurodegenerative diseases. Clustered regularly-interspaced short palindromic repeats (CRISPR) is the latest technology for genome editing. Being simple to use and highly efficient, CRISPR-based genome-editing tools are rapidly gaining popularity in biomedical research and opening up new avenues for disease treatment. Here, we review the development of CRISPR-based genome-editing tools and their applications in HD research to offer a translational perspective on advancing the genome-editing technology to HD treatment.




Similar content being viewed by others
References
Lieberman AP, Shakkottai VG, Albin RL. Polyglutamine repeats in neurodegenerative diseases. Annu Rev Pathol 2019, 14: 1–27.
Zuccato C, Valenza M, Cattaneo E. Molecular mechanisms and potential therapeutical targets in Huntington’s disease. Physiol Rev 2010, 90: 905–981.
Lu SY, Lu BX. Degeneration versus development: Hunting-out the D-unit of Huntington’s disease. Neurosci Bull 2021, 37: 757–760.
Cheng HR, Li XY, Yu HL, Xu M, Zhang YB, Gan SR. Correlation between CCG polymorphisms and CAG repeats during germline transmission in Chinese patients with Huntington’s disease. Neurosci Bull 2020, 36: 811–814.
Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci 2007, 30: 575–621.
Bates GP, Dorsey R, Gusella JF, Hayden MR, Kay C, Leavitt BR, et al. Huntington disease. Nat Rev Dis Primers 2015, 1: 15005.
A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell 1993, 72: 971–983. doi: https://doi.org/10.1016/0092-8674(93)90585-e.
Carroll D. Genome engineering with targetable nucleases. Annu Rev Biochem 2014, 83: 409–439.
Hockemeyer D, Soldner F, Beard C, Gao Q, Mitalipova M, DeKelver RC, et al. Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nat Biotechnol 2009, 27: 851–857.
Gaj T, Gersbach CA, Barbas CF III. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol 2013, 31: 397–405.
Vachey G, Déglon N. CRISPR/Cas9-mediated genome editing for Huntington’s disease. Methods Mol Biol 2018, 1780: 463–481.
Ishino Y, Shinagawa H, Makino K, Amemura M, Nakata A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J Bacteriol 1987, 169: 5429–5433.
Mojica FJ, Ferrer C, Juez G, Rodríguez-Valera F. Long stretches of short tandem repeats are present in the largest replicons of the Archaea Haloferax mediterranei and Haloferax volcanii and could be involved in replicon partitioning. Mol Microbiol 1995, 17: 85–93.
Jansen R, Embden JD, Gaastra W, Schouls LM. Identification of genes that are associated with DNA repeats in prokaryotes. Mol Microbiol 2002, 43: 1565–1575.
Horvath P, Barrangou R. CRISPR/Cas, the immune system of bacteria and Archaea. Science 2010, 327: 167–170.
Bolotin A, Quinquis B, Sorokin A, Ehrlich SD. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology (Reading) 2005, 151: 2551–2561.
Pourcel C, Salvignol G, Vergnaud G. CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies. Microbiology (Reading) 2005, 151: 653–663.
Mojica FJM, Díez-Villaseñor C, García-Martínez J, Soria E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J Mol Evol 2005, 60: 174–182.
Makarova KS, Grishin NV, Shabalina SA, Wolf YI, Koonin EV. A putative RNA-interference-based immune system in prokaryotes: Computational analysis of the predicted enzymatic machinery, functional analogies with eukaryotic RNAi, and hypothetical mechanisms of action. Biol Direct 2006, 1: 7.
Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science 2007, 315: 1709–1712.
Andersson AF, Banfield JF. Virus population dynamics and acquired virus resistance in natural microbial communities. Science 2008, 320: 1047–1050.
Brouns SJJ, Jore MM, Lundgren M, Westra ER, Slijkhuis RJH, Snijders APL, et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science 2008, 321: 960–964.
Garneau JE, Dupuis MÈ, Villion M, Romero DA, Barrangou R, Boyaval P, et al. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 2010, 468: 67–71.
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337: 816–821.
Makarova KS, Wolf YI, Koonin EV. Classification and nomenclature of CRISPR-cas systems: Where from here? CRISPR J 2018, 1: 325–336.
Adli M. The CRISPR tool kit for genome editing and beyond. Nat Commun 1911, 2018: 9.
Nishiyama J, Mikuni T, Yasuda R. Virus-mediated genome editing via homology-directed repair in mitotic and postmitotic cells in mammalian brain. Neuron 2017, 96: 755-768.e5.
Mao ZY, Bozzella M, Seluanov A, Gorbunova V. DNA repair by nonhomologous end joining and homologous recombination during cell cycle in human cells. Cell Cycle 2008, 7: 2902–2906.
Bekris LM, Yu CE, Bird TD, Tsuang DW. Genetics of Alzheimer disease. J Geriatr Psychiatry Neurol 2010, 23: 213–227.
Klein C, Westenberger A. Genetics of Parkinson’s disease. Cold Spring Harb Perspect Med 2012, 2: a008888.
Mittelman D, Moye C, Morton J, Sykoudis K, Lin YF, Carroll D, et al. Zinc-finger directed double-strand breaks within CAG repeat tracts promote repeat instability in human cells. Proc Natl Acad Sci U S A 2009, 106: 9607–9612.
Garriga-Canut M, Agustín-Pavón C, Herrmann F, Sánchez A, Dierssen M, Fillat C, et al. Synthetic zinc finger repressors reduce mutant huntingtin expression in the brain of R6/2 mice. Proc Natl Acad Sci U S A 2012, 109: E3136–E3145.
Fink KD, Deng P, Gutierrez J, Anderson JS, Torrest A, Komarla A, et al. Allele-specific reduction of the mutant huntingtin allele using transcription activator-like effectors in human Huntington’s disease fibroblasts. Cell Transplant 2016, 25: 677–686.
Cong L, Ran FA, Cox D, Lin SL, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339: 819–823.
An MC, O'Brien RN, Zhang NZ, Patra BN, de la Cruz M, Ray A, et al. Polyglutamine disease modeling: Epitope based screen for homologous recombination using CRISPR/Cas9 system. PLoS Curr 2014, https://doi.org/10.1371/currents.hd.0242d2e7ad72225efa72f6964589369a
Malankhanova T, Suldina L, Grigor’eva E, Medvedev S, Minina J, Morozova K, et al. A human induced pluripotent stem cell-derived isogenic model of Huntington’s disease based on neuronal cells has several relevant phenotypic abnormalities. J Pers Med 2020, 10: 215.
Xu XH, Tay Y, Sim B, Yoon SI, Huang YH, Ooi J, et al. Reversal of phenotypic abnormalities by CRISPR/Cas9-mediated gene correction in Huntington disease patient-derived induced pluripotent stem cells. Stem Cell Reports 2017, 8: 619–633.
Menalled LB, Kudwa AE, Miller S, Fitzpatrick J, Watson-Johnson J, Keating N, et al. Comprehensive behavioral and molecular characterization of a new knock-in mouse model of Huntington’s disease: zQ175. PLoS One 2012, 7: e49838.
Menalled LB, Sison JD, Dragatsis I, Zeitlin S, Chesselet MF. Time course of early motor and neuropathological anomalies in a knock-in mouse model of Huntington’s disease with 140 CAG repeats. J Comp Neurol 2003, 465: 11–26.
Lin CH, Tallaksen-Greene S, Chien WM, Cearley JA, Jackson WS, Crouse AB, et al. Neurological abnormalities in a knock-in mouse model of Huntington’s disease. Hum Mol Genet 2001, 10: 137–144.
Heng MY, Duong DK, Albin RL, Tallaksen-Greene SJ, Hunter JM, Lesort MJ, et al. Early autophagic response in a novel knock-in model of Huntington disease. Hum Mol Genet 2010, 19: 3702–3720.
Lee CYD, Cantle JP, Yang XW. Genetic manipulations of mutant huntingtin in mice: New insights into Huntington’s disease pathogenesis. FEBS J 2013, 280: 4382–4394.
Ross CA, Tabrizi SJ. Huntington’s disease: From molecular pathogenesis to clinical treatment. Lancet Neurol 2011, 10: 83–98.
Yang HM, Yang S, Jing L, Huang LX, Chen LX, Zhao XX, et al. Truncation of mutant huntingtin in knock-in mice demonstrates exon1 huntingtin is a key pathogenic form. Nat Commun 2020, 11: 2582.
Bañez-Coronel M, Ayhan F, Tarabochia AD, Zu T, Perez BA, Tusi SK, et al. RAN translation in Huntington disease. Neuron 2015, 88: 667–677.
Yang S, Yang HM, Huang LX, Chen LX, Qin ZH, Li SH, et al. Lack of RAN-mediated toxicity in Huntington’s disease knock-in mice. Proc Natl Acad Sci U S A 2020, 117: 4411–4417.
Crook ZR, Housman D. Huntington’s disease: Can mice lead the way to treatment? Neuron 2011, 69: 423–435.
Levine MS, Cepeda C, Hickey MA, Fleming SM, Chesselet MF. Genetic mouse models of Huntington’s and Parkinson’s diseases: Illuminating but imperfect. Trends Neurosci 2004, 27: 691–697.
Yan S, Tu ZC, Liu ZM, Fan NN, Yang HM, Yang S, et al. A huntingtin knockin pig model recapitulates features of selective neurodegeneration in Huntington’s disease. Cell 2018, 173: 989-1002.e13.
Culver BP, Savas JN, Park SK, Choi JH, Zheng SQ, Zeitlin SO, et al. Proteomic analysis of wild-type and mutant huntingtin-associated proteins in mouse brains identifies unique interactions and involvement in protein synthesis. J Biol Chem 2012, 287: 21599–21614.
Goehler H, Lalowski M, Stelzl U, Waelter S, Stroedicke M, Worm U, et al. A protein interaction network links GIT1, an enhancer of huntingtin aggregation, to Huntington’s disease. Mol Cell 2004, 15: 853–865.
Shirasaki DI, Greiner ER, Al-Ramahi I, Gray M, Boontheung P, Geschwind DH, et al. Network organization of the huntingtin proteomic interactome in mammalian brain. Neuron 2012, 75: 41–57.
Wertz MH, Mitchem MR, Pineda SS, Hachigian LJ, Lee H, Lau V, et al. Genome-wide in vivo CNS screening identifies genes that modify CNS neuronal survival and mHTT toxicity. Neuron 2020, 106: 76-89.e8.
Genetic Modifiers of Huntington’s Disease (GeM-HD) Consortium. Identification of genetic factors that modify clinical onset of Huntington's disease. Cell 2015, 162: 516–526.
Lee JM, Chao MJ, Harold D, Abu Elneel K, Gillis T, Holmans P, et al. A modifier of Huntington’s disease onset at the MLH1 locus. Hum Mol Genet 2017, 26: 3859–3867.
Loupe JM, Pinto RM, Kim KH, Gillis T, Mysore JS, Andrew MA, et al. Promotion of somatic CAG repeat expansion by Fan1 knock-out in Huntington’s disease knock-in mice is blocked by Mlh1 knock-out. Hum Mol Genet 2020, 29: 3044–3053.
Li XJ, Li SH, Sharp AH, Nucifora FC, Schilling G, Lanahan A, et al. A huntingtin-associated protein enriched in brain with implications for pathology. Nature 1995, 378: 398–402.
Liu Q, Cheng SY, Yang HM, Zhu LY, Pan YC, Jing L, et al. Loss of Hap1 selectively promotes striatal degeneration in Huntington disease mice. Proc Natl Acad Sci U S A 2020, 117: 20265–20273.
Zhao T, Hong Y, Yin P, Li SH, Li XJ. Differential HspBP1 expression accounts for the greater vulnerability of neurons than astrocytes to misfolded proteins. Proc Natl Acad Sci U S A 2017, 114: E7803–E7811.
Drouet V, Ruiz M, Zala D, Feyeux M, Auregan G, Cambon K, et al. Allele-specific silencing of mutant huntingtin in rodent brain and human stem cells. PLoS One 2014, 9: e99341.
Kingwell K. Double setback for ASO trials in Huntington disease. Nat Rev Drug Discov 2021, 20: 412–413.
Li ZY, Wang C, Wang ZY, Zhu CG, Li J, Sha T, et al. Allele-selective lowering of mutant HTT protein by HTT-LC3 linker compounds. Nature 2019, 575: 203–209.
Shin JW, Kim KH, Chao MJ, Atwal RS, Gillis T, MacDonald ME, et al. Permanent inactivation of Huntington’s disease mutation by personalized allele-specific CRISPR/Cas9. Hum Mol Genet 2016, 25: 4566–4576.
Monteys AM, Ebanks SA, Keiser MS, Davidson BL. CRISPR/Cas9 editing of the mutant huntingtin allele in vitro and in vivo. Mol Ther 2017, 25: 12–23.
Grondin R, Kaytor MD, Ai Y, Nelson PT, Thakker DR, Heisel J, et al. Six-month partial suppression of Huntingtin is well tolerated in the adult rhesus striatum. Brain 2012, 135: 1197–1209.
McBride JL, Pitzer MR, Boudreau RL, Dufour B, Hobbs T, Ojeda SR, et al. Preclinical safety of RNAi-mediated HTT suppression in the rhesus macaque as a potential therapy for Huntington’s disease. Mol Ther 2011, 19: 2152–2162.
Wang GH, Liu XD, Gaertig MA, Li SH, Li XJ. Ablation of huntingtin in adult neurons is nondeleterious but its depletion in young mice causes acute pancreatitis. Proc Natl Acad Sci U S A 2016, 113: 3359–3364.
Yang S, Chang RB, Yang HM, Zhao T, Hong Y, Kong HE, et al. CRISPR/Cas9-mediated gene editing ameliorates neurotoxicity in mouse model of Huntington’s disease. J Clin Invest 2017, 127: 2719–2724.
Ekman FK, Ojala DS, Adil MM, Lopez PA, Schaffer DV, Gaj T. CRISPR-Cas9-mediated genome editing increases lifespan and improves motor deficits in a Huntington’s disease mouse model. Mol Ther Nucleic Acids 2019, 17: 829–839.
Yang S, Li SH, Li XJ. Shortening the half-life of Cas9 maintains its gene editing ability and reduces neuronal toxicity. Cell Rep 2018, 25: 2653-2659.e3.
Merienne N, Vachey G, de Longprez L, Meunier C, Zimmer V, Perriard G, et al. The self-inactivating KamiCas9 system for the editing of CNS disease genes. Cell Rep 2017, 20: 2980–2991.
Lopes C, Tang Y, Anjo SI, Manadas B, Onofre I, de Almeida LP, et al. Mitochondrial and redox modifications in Huntington disease induced pluripotent stem cells rescued by CRISPR/Cas9 CAGs targeting. Front Cell Dev Biol 2020, 8: 576592.
Chew WL, Tabebordbar M, Cheng JKW, Mali P, Wu EY, Ng AHM, et al. A multifunctional AAV-CRISPR-Cas9 and its host response. Nat Methods 2016, 13: 868–874.
Wang D, Mou HW, Li SY, Li YX, Hough S, Tran K, et al. Adenovirus-mediated somatic genome editing of pten by CRISPR/Cas9 in mouse liver in spite of Cas9-specific immune responses. Hum Gene Ther 2015, 26: 432–442.
Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, Essletzbichler P, et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 2015, 163: 759–771.
Zetsche B, Heidenreich M, Mohanraju P, Fedorova I, Kneppers J, DeGennaro EM, et al. Multiplex gene editing by CRISPR-Cpf1 using a single crRNA array. Nat Biotechnol 2017, 35: 31–34.
Kim E, Koo T, Park SW, Kim D, Kim K, Cho HY, et al. In vivo genome editing with a small Cas9 orthologue derived from Campylobacter jejuni. Nat Commun 2017, 8: 14500.
Harrington LB, Burstein D, Chen JS, Paez-Espino D, Ma EB, Witte IP, et al. Programmed DNA destruction by miniature CRISPR-Cas14 enzymes. Science 2018, 362: 839–842.
Xiao RJ, Li Z, Wang SK, Han RJ, Chang LF. Structural basis for substrate recognition and cleavage by the dimerization-dependent CRISPR-Cas12f nuclease. Nucleic Acids Res 2021, 49: 4120–4128.
Takeda SN, Nakagawa R, Okazaki S, Hirano H, Kobayashi K, Kusakizako T, et al. Structure of the miniature type V-F CRISPR-Cas effector enzyme. Mol Cell 2021, 81: 558-570.e3.
Koo T, Lu-Nguyen NB, Malerba A, Kim E, Kim D, Cappellari O, et al. Functional rescue of dystrophin deficiency in mice caused by frameshift mutations using Campylobacter jejuni Cas9. Mol Ther 2018, 26: 1529–1538.
Lee JY, Jang YJ, Bae JH, Lee YH, Bae HS, Kim S, et al. Efficient and specific generation of knockout mice using Campylobacter jejuni CRISPR/Cas9 system. Biochem Biophys Rep 2020, 22: 100752.
Bigelyte G, Young JK, Karvelis T, Budre K, Zedaveinyte R, Djukanovic V, et al. Miniature type V-F CRISPR-Cas nucleases enable targeted DNA modification in cells. Nat Commun 2021, 12: 6191.
Kim DY, Lee JM, Moon SB, Chin HJ, Park S, Lim Y, et al. Efficient CRISPR editing with a hypercompact Cas12f1 and engineered guide RNAs delivered by adeno-associated virus. Nat Biotechnol 2022, 40: 94–102.
Nelles DA, Fang MY, O’Connell MR, Xu JL, Markmiller SJ, Doudna JA, et al. Programmable RNA tracking in live cells with CRISPR/Cas9. Cell 2016, 165: 488–496.
Batra R, Nelles DA, Pirie E, Blue SM, Marina RJ, Wang H, et al. Elimination of toxic microsatellite repeat expansion RNA by RNA-targeting Cas9. Cell 2017, 170: 899-912.e10.
Goell JH, Hilton IB. CRISPR/cas-based epigenome editing: Advances, applications, and clinical utility. Trends Biotechnol 2021, 39: 678–691.
Maeder ML, Linder SJ, Cascio VM, Fu YF, Ho QH, Joung JK. CRISPR RNA-guided activation of endogenous human genes. Nat Methods 2013, 10: 977–979.
Gilbert LA, Larson MH, Morsut L, Liu ZR, Brar GA, Torres SE, et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 2013, 154: 442–451.
Amabile A, Migliara A, Capasso P, Biffi M, Cittaro D, Naldini L, et al. Inheritable silencing of endogenous genes by hit-and-Run targeted epigenetic editing. Cell 2016, 167: 219-232.e14.
Liu XS, Wu H, Ji X, Stelzer Y, Wu XB, Czauderna S, et al. Editing DNA methylation in the mammalian genome. Cell 2016, 167: 233-247.e17.
Hilton IB, D’Ippolito AM, Vockley CM, Thakore PI, Crawford GE, Reddy TE, et al. Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat Biotechnol 2015, 33: 510–517.
Kwon DY, Zhao YT, Lamonica JM, Zhou ZL. Locus-specific histone deacetylation using a synthetic CRISPR-Cas9-based HDAC. Nat Commun 2017, 8: 15315.
Zeitler B, Froelich S, Marlen K, Shivak DA, Yu Q, Li D, et al. Allele-selective transcriptional repression of mutant HTT for the treatment of Huntington’s disease. Nat Med 2019, 25: 1131–1142.
Gaudelli NM, Komor AC, Rees HA, Packer MS, Badran AH, Bryson DI, et al. Programmable base editing of A·T to G·C in genomic DNA without DNA cleavage. Nature 2017, 551: 464–471.
Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016, 533: 420–424.
Iyama T, Wilson DM III. DNA repair mechanisms in dividing and non-dividing cells. DNA Repair 2013, 12: 620–636.
Kuscu C, Parlak M, Tufan TR, Yang JK, Szlachta K, Wei XL, et al. CRISPR-STOP: Gene silencing through base-editing-induced nonsense mutations. Nat Methods 2017, 14: 710–712.
Lim CKW, Gapinske M, Brooks AK, Woods WS, Powell JE, et al. Treatment of a mouse model of ALS by in vivo base editing. Mol Ther 2020, 28: 1177–1189.
Kolli N, Lu M, Maiti P, Rossignol J, Dunbar GL. CRISPR-Cas9 mediated gene-silencing of the mutant huntingtin gene in an in vitro model of Huntington’s disease. Int J Mol Sci 2017, 18: 754.
Funding
This review was supported by the National Key R&D Program of China (2021YFA0805200), the National Natural Science Foundation of China (31970954, 81901289 and 31872779) and the Guangdong Key Laboratory of Non-human Primate Research (2020B121201006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Qin, Y., Li, S., Li, XJ. et al. CRISPR-Based Genome-Editing Tools for Huntington’s Disease Research and Therapy. Neurosci. Bull. 38, 1397–1408 (2022). https://doi.org/10.1007/s12264-022-00880-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-022-00880-3