Abstract
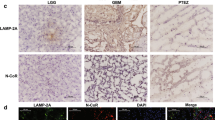
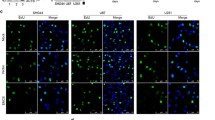
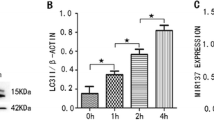
Previous studies suggest that the reduction of SMAD3 (mothers against decapentaplegic homolog 3) has a great impact on tumor development, but its exact pathological function remains unclear. In this study, we found that the protein level of SMAD3 was greatly reduced in human-grade IV glioblastoma tissues, in which LAMP2A (lysosome-associated membrane protein type 2A) was significantly up-regulated. LAMP2A is a key rate-limiting protein of chaperone-mediated autophagy (CMA), a lysosome pathway of protein degradation that is activated in glioma. We carefully analyzed the amino-acid sequence of SMAD3 and found that it contained a pentapeptide motif biochemically related to KFERQ, which has been proposed to be a targeting sequence for CMA. In vitro, we confirmed that SMAD3 was degraded in either serum-free or KFERQ motif deleted condition, which was regulated by LAMP2A and interacted with HSC70 (heat shock cognate 71 kDa protein). Using isolated lysosomes, amino-acid residues 75 and 128 of SMAD3 were found to be of importance for this process, which affected the CMA pathway in which SMAD3 was involved. Similarly, down-regulating SMAD3 or up-regulating LAMP2A in cultured glioma cells enhanced their proliferation and invasion. Taken together, these results suggest that excessive activation of CMA regulates glioma cell growth by promoting the degradation of SMAD3. Therefore, targeting the SMAD3–LAMP2A-mediated CMA-lysosome pathway may be a promising approach in anti-cancer therapy.





Similar content being viewed by others
References
Jacob F, Salinas RD, Zhang DY, Nguyen PTT, Schnoll JG, Wong SZH. A patient-derived glioblastoma organoid model and biobank recapitulates inter- and intra-tumoral heterogeneity. Cell 2020, 180: 188-204.e22.
Müller Bark J, Kulasinghe A, Chua B, Day BW, Punyadeera C. Circulating biomarkers in patients with glioblastoma. Br J Cancer 2020, 122: 295–305.
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol 2016, 131: 803–820.
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathologica 2007, 114: 97–109.
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005, 352: 987–996.
Jackson CM, Choi J, Lim M. Mechanisms of immunotherapy resistance: Lessons from glioblastoma. Nat Immunol 2019, 20: 1100–1109.
Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol 2005, 21: 659–693.
Shi YG, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003, 113: 685–700.
Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol 2012, 13: 616–630.
Kjellman C, Olofsson SP, Hansson O, von Schantz T, Lindvall M, Nilsson I, et al. Expression of TGF-beta isoforms, TGF-beta receptors, and SMAD molecules at different stages of human glioma. Int J Cancer 2000, 89: 251–258.
Zhang L, Sato E, Amagasaki K, Nakao A, Naganuma H. Participation of an abnormality in the transforming growth factor-beta signaling pathway in resistance of malignant glioma cells to growth inhibition induced by that factor. J Neurosurg 2006, 105: 119–128.
Kaminska B, Kocyk M, Kijewska M. TGF beta signaling and its role in glioma pathogenesis. Adv Exp Med Biol 2013, 986: 171–187.
Dong CY, Mi RF, Jin GS, Zhou YQ, Zhang J, Liu FS. Identification of the proliferative effect of Smad2 and 3 in the TGF β2/Smad signaling pathway using RNA interference in a glioma cell line. Mol Med Rep 2015, 12: 1824–1828.
Kaushik S, Cuervo AM. The coming of age of chaperone-mediated autophagy. Nat Rev Mol Cell Biol 2018, 19: 365–381.
Kaushik S, Cuervo AM. Chaperone-mediated autophagy: A unique way to enter the lysosome world. Trends Cell Biol 2012, 22: 407–417.
Patel B, Cuervo AM. Methods to study chaperone-mediated autophagy. Methods 2015, 75: 133–140.
Wang RB, Liu YT, Liu L, Chen M, Wang XX, Yang JY, et al. Tumor cells induce LAMP2a expression in tumor-associated macrophage for cancer progression. EBioMedicine 2019, 40: 118–134.
Saha T. LAMP2A overexpression in breast tumors promotes cancer cell survival via chaperone-mediated autophagy. Autophagy 2012, 8: 1643–1656.
Vakifahmetoglu-Norberg H, Kim M, Xia HG, Iwanicki MP, Ofengeim D, Coloff JL, et al. Corrigendum: Chaperone-mediated autophagy degrades mutant p53. Genes Dev 2016, 30: 870.
Kon M, Kiffin R, Koga H, Chapochnick J, MacIan F, Varticovski L, et al. Chaperone-mediated autophagy is required for tumor growth. Sci Transl Med 2011, 3: 109ra117.
Zhou JF, Yang JJ, Fan X, Hu SJ, Zhou FL, Dong JQ, et al. Chaperone-mediated autophagy regulates proliferation by targeting RND3 in gastric cancer. Autophagy 2016, 12: 515–528.
Suzuki J, Nakajima W, Suzuki H, Asano Y, Tanaka N. Chaperone-mediated autophagy promotes lung cancer cell survival through selective stabilization of the pro-survival protein, MCL1. Biochem Biophys Res Commun 2017, 482: 1334–1340.
Welsch T, Younsi A, Disanza A, Rodriguez JA, Cuervo AM, Scita G, et al. Eps8 is recruited to lysosomes and subjected to chaperone-mediated autophagy in cancer cells. Exp Cell Res 2010, 316: 1914–1924.
Ding ZB, Fu XT, Shi YH, Zhou J, Peng YF, Liu WR, et al. Lamp2a is required for tumor growth and promotes tumor recurrence of hepatocellular carcinoma. Int J Oncol 2016, 49: 2367–2376.
Wen X, Klionsky DJ. At a glance: A history of autophagy and cancer. Semin Cancer Biol 2020, 66: 3–11.
Ali AB, Nin DS, Tam J, Khan M. Role of chaperone mediated autophagy (CMA) in the degradation of misfolded N-CoR protein in non-small cell lung cancer (NSCLC) cells. PLoS One 2011, 6: e25268.
Hao YQ, Kacal M, Ouchida AT, Zhang BX, Norberg E, Vakifahmetoglu-Norberg H. Targetome analysis of chaperone-mediated autophagy in cancer cells. Autophagy 2019, 15: 1558–1571.
Aguado C, Pérez-Jiménez E, Lahuerta M, Knecht E. Isolation of lysosomes from mammalian tissues and cultured cells. Methods Mol Biol 2016, 1449: 299–311.
Catarino S, Pereira P, Girão H. Molecular control of chaperone-mediated autophagy. Essays Biochem 2017, 61: 663–674.
Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: A clinical review. JAMA 2013, 310: 1842–1850.
Jiang Z, Guo Y, Miao LF, Han LZ, Zhang W, Jiang YQ. SMAD3 silencing enhances DNA damage in radiation therapy by interacting with MRE11-RAD50-NBS1 complex in glioma. J Biochem 2019, 165: 317–322.
Dice JF. Microinjected ribonuclease A as a probe for lysosomal pathways of intracellular protein degradation. J Protein Chem 1988, 7: 115–127.
Li ZY, Li CZ, Wu YT, Lan XL, Cao L, Hong LC, et al. The expression of TGF-β1, Smad3, phospho-Smad3 and Smad7 is correlated with the development and invasion of nonfunctioning pituitary adenomas. J Transl Med 2014, 12: 71.
Massagué J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell 2000, 103: 295–309.
Tang LY, Yamashita M, Coussens NP, Tang Y, Wang XC, Li CL, et al. Ablation of Smurf2 reveals an inhibition in TGF-β signalling through multiple mono-ubiquitination of Smad3. EMBO J 2011, 30: 4777–4789.
Gao S, Alarcón C, Sapkota G, Rahman S, Chen PY, Goerner N, et al. Ubiquitin ligase Nedd4L targets activated Smad2/3 to limit TGF-beta signaling. Mol Cell 2009, 36: 457–468.
Lu Z, Li X, Xu Y, Chen M, Chen W, Chen T, et al. microRNA-17 functions as an oncogene by downregulating Smad3 expression in hepatocellular carcinoma. Cell Death Dis 2019, 10: 723.
Liao ZB, Chen L, Zhang XW, Zhang HW, Tan XL, Dong KS, et al. PTPRε Acts as a metastatic promoter in hepatocellular carcinoma by facilitating recruitment of SMAD3 to TGF-β receptor 1. Hepatology 2020, 72: 997–1012.
Liu S, Ren J, Ten Dijke P. Targeting TGFβ signal transduction for cancer therapy. Signal Transduct Target Ther 2021, 6: 8.
Ooshima A, Park J, Kim SJ. Phosphorylation status at Smad3 linker region modulates transforming growth factor-β-induced epithelial-mesenchymal transition and cancer progression. Cancer Sci 2019, 110: 481–488.
Tarasewicz E, Jeruss JS. Phospho-specific Smad3 signaling: Impact on breast oncogenesis. Cell Cycle 2012, 11: 2443–2451.
Niu H, Huang YW, Yan L, Zhang L, Zhao MN, Lu T, et al. Knockdown of SMAD3 inhibits the growth and enhances the radiosensitivity of lung adenocarcinoma via p21 in vitro and in vivo. Int J Biol Sci 2020, 16: 1010–1022.
Yokoyama S, Iwakami Y, Hang Z, Kin R, Zhou Y, Yasuta Y, et al. Targeting PSMD14 inhibits melanoma growth through SMAD3 stabilization. Sci Rep 2020, 10: 19214.
Jiang L, Wang RR, Fang L, Ge XL, Chen LN, Zhou M, et al. HCP5 is a SMAD3-responsive long non-coding RNA that promotes lung adenocarcinoma metastasis via miR-203/SNAI axis. Theranostics 2019, 9: 2460–2474.
Gong YF, Li D, Li LL, Yang J, Ding HY, Zhang C, et al. Smad3 C-terminal phosphorylation site mutation attenuates the hepatoprotective effect of salvianolic acid B against hepatocarcinogenesis. Food Chem Toxicol 2021, 147: 111912.
Fleming NI, Jorissen RN, Mouradov D, Christie M, Sakthianandeswaren A, Palmieri M, et al. SMAD2, SMAD3 and SMAD4 mutations in colorectal cancer. Cancer Res 2013, 73: 725–735.
Shi YL, Wang XY, Xu ZY, He Y, Guo CY, He LJ, et al. PDLIM5 inhibits STUB1-mediated degradation of SMAD3 and promotes the migration and invasion of lung cancer cells. J Biol Chem 2020, 295: 13798–13811.
Li X, He S, Ma B. Autophagy and autophagy-related proteins in cancer. Mol Cancer 2020, 19: 12.
Xia HG, Najafov A, Geng JF, Galan-Acosta L, Han XM, Guo Y, et al. Degradation of HK2 by chaperone-mediated autophagy promotes metabolic catastrophe and cell death. J Cell Biol 2015, 210: 705–716.
Cuervo AM, Wong E. Chaperone-mediated autophagy: Roles in disease and aging. Cell Res 2014, 24: 92–104.
Alvarez-Erviti L, Seow Y, Schapira AH, Rodriguez-Oroz MC, Obeso JA, Cooper JM. Influence of microRNA deregulation on chaperone-mediated autophagy and α-synuclein pathology in Parkinson’s disease. Cell Death Dis 2013, 4: e545.
Acknowledgments
The authors would like to thank Professors Qian Yang and Pingyi Xu for two years of patient guidance. This work was supported by the National Key R&D Program of China (2016YFC1306601 and 2017YFC1306002), the National Natural Science Foundation of China (82071416, 81870992, 81870856, U1603281, 81903958, 81901282, and 82004459), the Natural Science Foundation of Guangdong Province (2020A1515010986, 2019A1515011189, and 2018A030310521), a Science and Technology Planning Project of Guangdong Province (A2018315), a Technology Project of Guangzhou (2018-1202-SF-0019 and 2019ZD09), and a China Postdoctoral Science Foundation Grant (2019M662873).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors claim that they do not have any potential conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, H., Yong, Y., Li, X. et al. Chaperone-mediated Autophagy Regulates Cell Growth by Targeting SMAD3 in Glioma. Neurosci. Bull. 38, 637–651 (2022). https://doi.org/10.1007/s12264-022-00818-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-022-00818-9