Abstract
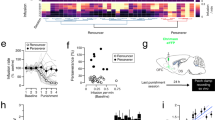
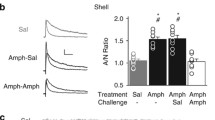
Plasticity in the glutamatergic synapses on striatal medium spiny neurons (MSNs) is not only essential for behavioral adaptation but also extremely vulnerable to drugs of abuse. Modulation on these synapses by even a single exposure to an addictive drug may interfere with the plasticity required by behavioral learning and thus produce impairment. In the present work, we found that the negative reinforcement learning, escaping mild foot-shocks by correct nose-poking, was impaired by a single in vivo exposure to 20 mg/kg cocaine 24 h before the learning in mice. Either a single exposure to cocaine or reinforcement learning potentiates the glutamatergic synapses on MSNs expressing the striatal dopamine 1 (D1) receptor (D1-MSNs). However, 24 h after the cocaine exposure, the potentiation required for reinforcement learning was disrupted. Specific manipulation of the activity of striatal D1-MSNs in D1-cre mice demonstrated that activation of these MSNs impaired reinforcement learning in normal D1-cre mice, but inhibition of these neurons reversed the reinforcement learning impairment induced by cocaine. The results suggest that cocaine potentiates the activity of direct pathway neurons in the dorsomedial striatum and this potentiation might disrupt the potentiation produced during and required for reinforcement learning.





Similar content being viewed by others
References
Verdejo-García A, Bechara A, Recknor EC, Pérez-García M. Executive dysfunction in substance dependent individuals during drug use and abstinence: An examination of the behavioral, cognitive and emotional correlates of addiction. J Int Neuropsychol Soc 2006, 12: 405–415.
Verdejo-García A, Pérez-García M. Profile of executive deficits in cocaine and heroin polysubstance users: Common and differential effects on separate executive components. Psychopharmacology (Berl) 2007, 190: 517–530.
Franken IHA, van Strien JW, Franzek EJ, van de Wetering BJ. Error-processing deficits in patients with cocaine dependence. Biol Psychol 2007, 75: 45–51.
Monterosso J, Ehrman R, Napier KL, O’Brien CP, Childress AR. Three decision-making tasks in cocaine-dependent patients: do they measure the same construct?. Addiction 2001, 96: 1825–1837.
Soar K, Mason C, Potton A, Dawkins L. Neuropsychological effects associated with recreational cocaine use. Psychopharmacology (Berl) 2012, 222(4): 633–643.
Bolla KI, Rothman R, Cadet JL. Dose-related neurobehavioral effects of chronic cocaine use. J Neuropsychiatry Clin Neurosci 1999, 11: 361–369.
Hoff AL, Riordan H, Morris L, Cestaro V, Wieneke M, Alpert R. Effects of crack cocaine on neurocognitive function. Psychiatry Res 1996, 60: 167–176.
Colzato LS, van den Wildenberg WPM, Hommel B. Impaired inhibitory control in recreational cocaine users. PLoS One 2007, 2: e1143.
Colzato LS, Hommel B. Recreational use of cocaine eliminates inhibition of return. Neuropsychology 2009, 23: 125–129.
Lüscher C. Cocaine-evoked synaptic plasticity of excitatory transmission in the ventral tegmental area. Cold Spring Harb Perspect Med 2013, 3: a012013.
Kasanetz F, Gamonet VD, Berson N, Balado E, Lafourcade M, Manzoni O, et al. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science 2010, 328: 1709–1712.
Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 2001, 411: 583–587.
Jin X, Tecuapetla F, Costa RM. Basal ganglia subcircuits distinctively encode the parsing and concatenation of action sequences. Nat Neurosci 2014, 17(3): 423–430.
Tecuapetla F, Jin X, Lima SQ, Costa RM. Complementary contributions of striatal projection pathways to action initiation and execution. Cell 2016, 166: 703–715.
Zhang CC, Ni PY, Liu YK, Tian Y, Wei JX, Xiang N, et al. GABAergic abnormalities associated with aensorimotor cortico-striatal community structural deficits in ErbB4 knockout mice and first-episode treatment-naïve patients with schizophrenia. Neurosci Bull 2020, 36: 97–109.
Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron 2010, 66: 896–907.
Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Hervé D, Valjent E, et al. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci 2008, 28: 5671–5685.
Beurrier C, Malenka RC. Enhanced inhibition of synaptic transmission by dopamine in the nucleus accumbens during behavioral sensitization to cocaine. J Neurosci 2002, 22: 5817–5822.
Calipari ES, Bagot RC, Purushothaman I, Davidson TJ, Yorgason IT, Pena CJ, et al. In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proc Natl Acad Sci USA 2016, 113: 2726–2731.
Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci 2012, 15: 816–818.
Costa RM. Plastic corticostriatal circuits for action learning: What’s dopamine got to do with it?. Ann N Y Acad Sci 2007, 1104: 172–191.
Costa RM, Cohen D, Nicolelis MAL. Differential corticostriatal plasticity during fast and slow motor skill learning in mice. Curr Biol 2004, 14: 1124–1134.
Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature 2005, 437: 1158–1161.
Yin HH, Mulcare SP, Hilário MRF, Clouse E, Hollowa T, Davis MI, et al. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci 2009, 12: 333–341.
Shan Q, Ge M, Christie MJ, Balleine BW. The acquisition of goal-directed actions generates opposing plasticity in direct and indirect pathways in dorsomedial striatum. J Neurosci 2014, 34: 9196–9201.
Kim JA, Pollak KA, Hjelmstad GO, Fields HL. A single cocaine exposure enhances both opioid reward and aversion through a ventral tegmental area-dependent mechanism. Proc Natl Acad Sci 2004, 101: 5664–5669.
Arbuthnott GW, Wickens J. Space, time and dopamine. Trends Neurosci 2007, 30: 62–69.
Zhu F, Wu Q, Li J, Grycel K, Liu B, Sun X, et al. A single dose of cocaine potentiates glutamatergic synaptic transmission onto locus coeruleus neurons. Cell Calcium 2017, 67: 11–20.
Volkow N, Li T. Science and society: drug addiction: the neurobiology of behaviour gone awry. Nat Rev Neurosci 2004, 5(12): 963–970.
Ferrario CR, Li X, Wolf ME. Effects of acute cocaine or dopamine receptor agonists on AMPA receptor distribution in the rat nucleus accumbens. Synapse 2011, 65: 54–63.
Schroeder JA, McCafferty MR, Unterwald EM. Regulation of dynamin 2 and G protein-coupled receptor kinase 2 in rat nucleus accumbens during acute and repeated cocaine administration. Synapse 2009, 63: 863–870.
Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu Rev Neurosci 2006, 29: 565–598.
Laetitia L, Thompson ED, Claus SK, Mikulich G, Marie TB, Thomas C, et al. Negative reinforcement learning is affected in substance dependence. Drug Alcohol Depend 2012, 123: 84–90.
Colzato LS, Huizinga M, Hommel B. Recreational cocaine polydrug use impairs cognitive flexibility but not working memory. Psychopharmacology 2009, 207: 225–234.
Colzato LS, van den Wildenberg WPM, Hommel B. Reduced spontaneous eye blink rates in recreational cocaine users: Evidence for dopaminergic hypoactivity. PLoS One 2008, 3: 1–4.
Yao L, Li YF, Qian ZQ, Wu ML, Yang HF, Chen NJ, et al. Loss of control over mild aversive events produces significant helplessness in mice. Behav Brain Res 2019, 376: 112173.
Wang X, Qiao Y, Dai Z, Sui N, Shen F, Zhang J, et al. Medium spiny neurons of the anterior dorsomedial striatum mediate reversal learning in a cell-type-dependent manner. Brain Struct Funct 2019, 224: 419–434.
Ting JT, Daigle TL, Chen Q, Feng G. Acute brain slice methods for adult and aging animals: application of targeted patch clamp analysis and optogenetics. Methods Mol Biol 2014, 1183: 221–242.
Huang S, Uusisaari MY. Elevated temperature during slicing enhances acute slice preparation quality. Front Cell Neurosci 2013, 7: 1–8.
Cadwell CR, Palasantza A, Jiang X, Berens P, Deng Q, Yilmaz M, et al. Electrophysiological, transcriptomic and morphologic profiling of single neurons using Patch-seq. Nat Biotechnol 2016, 34: 199–203.
Cadwell CR, Scala F, Li S, Livrizzi G, Shen S, Sandberg R, et al. Multimodal profiling of single-cell morphology, electrophysiology, and gene expression using Patch-seq. Nat Protoc 2017, 12: 2531–2553.
Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Rev 1991, 16: 223–244.
Hikida T, Kaneko S, Isobe T, Kitabatake Y, Watanabe D, Pastan I, et al. Increased sensitivity to cocaine by cholinergic cell ablation in nucleus accumbens. Proc Natl Acad Sci 2001, 98: 13351–13354.
Liu L, Xu HF, Wang J, Li J, Tian YY, Zheng JQ, et al. Cell type-differential modulation of prefrontal cortical GABAergic interneurons on low gamma rhythm and social interaction. Sci Adv 2020, 6: 1–14.
Chen P, Lou SH, Huang ZH, Wang ZN, Shan QH, Wang Y, et al. Prefrontal cortex corticotropin-releasing factor neurons control behavioral style selection under challenging situations. Neuron 2020, 106: 301–315.
Wang WT, Li CC, Chen Q, Goes MS, Hawrot J, Yao AY, et al. Striatopallidal dysfunction underlies repetitive behavior in Shank3-deficient model of autism. J Clin Invest 2017, 127: 1978–1990.
Jin X, Costa RM. Shaping action sequences in basal ganglia circuits. Curr Opin Neurobiol 2015, 33: 188–196.
Santos FJ, Oliveira RF, Jin X, Costa RM. Corticostriatal dynamics encode the refinement of specific behavioral variability during skill learning. Elife 2015, 4: 1–19.
Sanders A, McDougall MG, Apodoca AMY, Adrian D, Mendez CG, Katz AT, et al. Ontogeny of cocaine-induced behaviors and cocaine pharmacokinetics in male and female neonatal, preweanling, and adult rats. Psychopharmacology (Berl) 2018, 235: 1967–1980.
Walsh SL, William W, Stoops DE. Moody SN, Lin GEB. Repeated dosing with oral cocaine in humans: assessment of direct effects, withdrawal and pharmacokinetics. Exp Clin Psychopharmacol 2009, 17: 205–216.
Terrier J, Lüscher C, Pascoli V. Cell-type specific insertion of GluA2-lacking AMPARs with cocaine exposure leading to sensitization, cue-induced seeking, and incubation of craving. Neuropsychopharmacology 2016, 41: 1779–1789.
Pascoli V, Turiault M, Lüscher C. Reversal of cocaine-evoked synaptic potentiation resets drug-induced adaptive behaviour. Nature 2011, 481: 71–76.
Willingham DB, Koroshetz WJ. Evidence for dissociable motor skills in Huntington’s disease patients. Psychobiology 1993, 21: 173–182.
Wichmann T, Delong MR. Functional neuroanatomy of the basal ganglia in Parkinson’s disease. Adv Neurol 2003, 91: 9–18.
Schimanski LA, Nguyen PV. Impaired fear memories are correlated with subregion-specific deficits in hippocampal and amygdalar LTP. Behav Neurosci 2005, 119: 38–54.
Shors TJ, Seib TB, Levine S, Thompson RF. Inescapable versus escapable shock modulates long-term potentiation in the rat hippocampus. Science 1989, 244: 224–226.
Brown MT, Bellone C, Mameli M, Labouèbe G, Bocklisch C, Balland B, et al. Drug-driven AMPA receptor redistribution mimicked by selective dopamine neuron stimulation. PLoS One 2010, 5.
Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 2003, 425: 917–925.
Heintz N. BAC to the future: The use of BAC transgenic mice for neuroscience research. Nat Rev Neurosci 2001, 2: 861–870.
Kramer PF, Christensen CH, Hazelwood LA, Dobi A, Alvarez VA. Dopamine D2 receptor overexpression alters behavior and physiology in Drd2-EGFP mice. J Neurosci 2011, 31: 126–132.
Ade KK, Wan Y, Chen M, Gloss B, Calakos N. An improved BAC transgenic fluorescent reporter line for sensitive and specific identification of striatonigral medium spiny neurons. Front Syst Neurosci 2011, 5: 1–9.
Matamales M, Bertran-Gonzalez J, Salomon L, Degos B, Deniau JM, Valjent E, et al. Striatal medium-sized spiny neurons: Identification by nuclear staining and study of neuronal subpopulations in BAC transgenic mice. PLoS One 2009, 4.
Hammad AM, Sari Y. Effects of cocaine exposure on astrocytic glutamate transporters and relapse-like ethanol-drinking behavior in male alcohol-preferring rats. Alcohol Alcohol 2020, 55: 254–263.
Buffalari DM, Rinaman L. Cocaine self-administration and extinction alter medullary noradrenergic and limbic forebrain cFos responses to acute, noncontingent cocaine injections in adult rats. Neuroscience 2014, 281: 241–250.
Lim TV, Cardinal RN, Savulich G, Jones PS, Moustafa AA, Robbins TW, et al. Impairments in reinforcement learning do not explain enhanced habit formation in cocaine use disorder. Psychopharmacology 2019, 236: 2359–2371.
Acknowledgments
We thank Dr. Fuqiang Xu for sharing the D1-Cre transgenic mice. This work was supported by he National Natural Science Foundation of China (81971285, 11727813), and the Fundamental Research Funds for the Central Universities (GK202005001), Shaanxi Normal University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Diao, Z., Di, Y., Wu, M. et al. Single Exposure to Cocaine Impairs Reinforcement Learning by Potentiating the Activity of Neurons in the Direct Striatal Pathway in Mice. Neurosci. Bull. 37, 1119–1134 (2021). https://doi.org/10.1007/s12264-021-00687-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-021-00687-8