Abstract
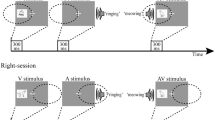
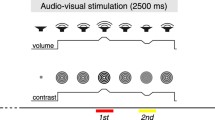
Cross-modal selective attention enhances the processing of sensory inputs that are most relevant to the task at hand. Such differential processing could be mediated by a swift network reconfiguration on the macroscopic level, but this remains a poorly understood process. To tackle this issue, we used a behavioral paradigm to introduce a shift of selective attention between the visual and auditory domains, and recorded scalp electroencephalographic signals from eight healthy participants. The changes in effective connectivity caused by the cross-modal attentional shift were delineated by analyzing spectral Granger Causality (GC), a metric of frequency-specific effective connectivity. Using data-driven methods of pattern-classification and feature-analysis, we found that a change in the α band (12 Hz–15 Hz) of GC is a stable feature across different individuals that can be used to decode the attentional shift. Specifically, auditory attention induces more pronounced information flow in the α band, especially from the parietal–occipital areas to the temporal–parietal areas, compared to the case of visual attention, reflecting a reconfiguration of interaction in the macroscopic brain network accompanying different processing. Our results support the role of α oscillation in organizing the information flow across spatially-separated brain areas and, thereby, mediating cross-modal selective attention.








Similar content being viewed by others
References
Driver J, Spence C. Cross-modal links in spatial attention. Philos Trans R Soc Lond B Biol Sci 1998, 353: 1319–1331.
Lavie N. Distracted and confused?: Selective attention under load. Trends Cogn Sci 2005, 9: 75–82.
Laurienti PJ, Burdette JH, Wallace MT, Yen YF, Field AS, Stein BE. Deactivation of sensory-specific cortex by cross-modal stimuli. J Cogn Neurosci 2002, 14: 420–429.
Rahne T, Bochmann M, von Specht H, Sussman ES. Visual cues can modulate integration and segregation of objects in auditory scene analysis. Brain Res 2007, 1144: 127–135.
Johnson JA, Zatorre RJ. Neural substrates for dividing and focusing attention between simultaneous auditory and visual events. Neuroimage 2006, 31: 1673–1681.
Shomstein S, Yantis S. Control of attention shifts between vision and audition in human cortex. J Neurosci 2004, 24: 10702–10706.
Calderone DJ, Lakatos P, Butler PD, Castellanos FX. Entrainment of neural oscillations as a modifiable substrate of attention. Trends Cogn Sci 2014, 18: 300–309.
Gomez-Ramirez M, Kelly SP, Molholm S, Sehatpour P, Schwartz TH, Foxe JJ. Oscillatory sensory selection mechanisms during intersensory attention to rhythmic auditory and visual inputs: a human electrocorticographic investigation. J Neurosci 2011, 31: 18556–18567.
Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science 2008, 320: 110–113.
Fiebelkorn IC, Kastner S. A rhythmic theory of attention. Trends Cogn Sci 2018.
Fiebelkorn IC, Foxe JJ, Butler JS, Mercier MR, Snyder AC, Molholm S. Ready, set, reset: stimulus-locked periodicity in behavioral performance demonstrates the consequences of cross-sensory phase reset. J Neurosci 2011, 31: 9971–9981.
Romei V, Gross J, Thut G. Sounds reset rhythms of visual cortex and corresponding human visual perception. Curr Biol 2012, 22: 807–813.
Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci 2009, 32: 9–18.
Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci 2010, 14: 506–515.
Buzsaki G, Watson BO. Brain rhythms and neural syntax: implications for efficient coding of cognitive content and neuropsychiatric disease. Dialogues Clin Neurosci 2012, 14: 345–367.
Allport A, Wylie G. Task switching, stimulus-response bindings, and negative priming. Cambridge: MIT Press, 2000.
Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science 2003, 302: 1181–1185.
Ding MZ, Bressler SL, Yang WM, Liang HL. Short-window spectral analysis of cortical event-related potentials by adaptive multivariate autoregressive modeling: data preprocessing, model validation, and variability assessment. Biol Cybern 2000, 83: 35–45.
Ding M, Chen Y, Bressler SL. Granger causality: basic theory and application to neuroscience. Handbook of time series analysis: recent theoretical developments and applications 2006: 437–460.
Seth AK. A MATLAB toolbox for Granger causal connectivity analysis. J Neurosci Methods 2010, 186: 262–273.
Richter CG, Coppola R, Bressler SL. Top-down beta oscillatory signaling conveys behavioral context in early visual cortex. Sci Rep 2018, 8: 12.
Geweke J. Measurement of linear-dependence and feedback between multiple time-series. J Am Stat Assoc 1982, 77: 304–313.
Bressler SL, Seth AK. Wiener-Granger Causality: A well established methodology. Neuroimage 2011, 58: 323–329.
Wolpaw JR, Birbaumer N, McFarland DJ, Pfurtscheller G, Vaughan TM. Brain-computer interfaces for communication and control. Clin Neurophysiol 2002, 113: 767–791.
Schirrmeister RT, Springenberg JT, Fiederer LDJ, Glasstetter M, Eggensperger K, Tangermann M, et al. Deep learning with convolutional neural networks for EEG decoding and visualization. Hum Brain Mapp 2017, 38: 5391–5420.
Muller KR, Tangermann M, Dornhege G, Krauledat M, Curio G, Blankertz B. Machine learning for real-time single-trial EEG-analysis: From brain-computer interfacing to mental state monitoring. J Neurosci Methods 2008, 167: 82–90.
Ramoser H, Muller-Gerking J, Pfurtscheller G. Optimal spatial filtering of single trial EEG during imagined hand movement. IEEE Trans Rehabil Eng 2000, 8: 441–446.
Chang C-C, Lin C-J. LIBSVM: A library for support vector machines. ACM transactions on intelligent systems and technology (TIST) 2011, 2: 27.
Breiman L. Arcing classifiers. Ann Stat 1998, 26: 801–824.
Guyon I, Weston J, Barnhill S, Vapnik V. Gene selection for cancer classification using support vector machines. Mach Learn 2002, 46: 389–422.
Siegel M, Donner TH, Oostenveld R, Fries P, Engel AK. Neuronal synchronization along the dorsal visual pathway reflects the focus of spatial attention. Neuron 2008, 60: 709–719.
van den Broek SP, Reinders F, Donderwinkel M, Peters M. Volume conduction effects in EEG and MEG. Electroencephalogr Clin Neurophysiol 1998, 106: 522–534.
Nunez PL, Srinivasan R, Westdorp AF, Wijesinghe RS, Tucker DM, Silberstein RB, et al. EEG coherency. 1. Statistics, reference electrode, volume conduction, Laplacians, cortical imaging, and interpretation at multiple scales. Electroencephalogr Clin Neurophysiol 1997, 103: 499–515.
Kaminski M, Blinowska KJ. Directed transfer function is not influenced by volume conduction-inexpedient pre-processing should be avoided. Front Comput Neurosci 2014, 8: 3.
Brunner C, Billinger M, Seeber M, Mullen TR, Makeig S. Volume conduction influences scalp-based connectivity estimates. Front Comput Neurosci 2016, 10: 4.
Kayser C, Petkov CI, Augath M, Logothetis NK. Functional imaging reveals visual modulation of specific fields in auditory cortex. J Neurosci 2007, 27: 1824–1835.
Sohrabpour A, Ye S, Worrell GA, Zhang WB, He B. Noninvasive electromagnetic source imaging and granger causality analysis: an electrophysiological connectome (econnectome) approach. IEEE Trans Biomed Eng 2016, 63: 2474–2487.
Michel CM, Murray MM, Lantz G, Gonzalez S, Spinelli L, de Peralta RG. EEG source imaging. Clin Neurophysiol 2004, 115: 2195–2222.
Tonin L, Leeb R, Millan JD. Time-dependent approach for single trial classification of covert visuospatial attention. J Neural Eng 2012, 9: 9.
Trachel RE, Clerc M, Brochier TG. Decoding covert shifts of attention induced by ambiguous visuospatial cues. Front Hum Neurosci 2015, 9: 9.
Treder MS, Bahramisharif A, Schmidt NM, van Gerven MAJ, Blankertz B. Brain-computer interfacing using modulations of alpha activity induced by covert shifts of attention. J NeuroEng Rehabil 2011, 8: 9.
Frey JN, Mainy N, Lachaux JP, Muller N, Bertrand O, Weisz N. Selective modulation of auditory cortical alpha activity in an audiovisual spatial attention task. J Neurosci. 2014, 34: 6634–6639.
Bagherzadeh Y, Baldauf D, Pantazis D, Desimone RJN. Alpha synchrony and the neurofeedback control of spatial attention. Neuron 2020, 105: 577–587.e5.
Foxe JJ, Snyder AC. The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention. Front Psychol 2011, 2: 154.
Fu KMG, Foxe JJ, Murray MM, Higgins BA, Javitt DC, Schroeder CE. Attention-dependent suppression of distracter visual input can be cross-modally cued as indexed by anticipatory parieto-occipital alpha-band oscillations. Cognit Brain Res 2001, 12: 145–152.
Gould IC, Rushworth MF, Nobre AC. Indexing the graded allocation of visuospatial attention using anticipatory alpha oscillations. J Neurophysiol 2011, 105: 1318–1326.
Rohenkohl G, Nobre AC. Alpha oscillations related to anticipatory attention follow temporal expectations. J Neurosci 2011, 31: 14076–14084.
Foxe JJ, Simpson GV, Ahlfors SP. Parieto-occipital similar to 10 Hz activity reflects anticipatory state of visual attention mechanisms. Neuroreport 1998, 9: 3929–3933.
Hong XF, Sun JF, Tong SB. Functional brain networks for sensory maintenance in top-down selective attention to audiovisual inputs. IEEE Trans. Neural Syst Rehabil Eng 2013, 21: 734–743.
Busse L, Roberts KC, Crist RE, Weissman DH, Woldorff MG. The spread of attention across modalities and space in a multisensory object. Proc Natl Acad Sci U S A 2005, 102: 18751–18756.
Degerman A, Rinne T, Pekkola J, Autti T, Jaaskelainen IP, Sams M, et al. Human brain activity associated with audiovisual perception and attention. Neuroimage 2007, 34: 1683–1691.
Johnson JA, Zatorre RJ. Attention to simultaneous unrelated auditory and visual events: Behavioral and neural correlates. Cereb Cortex 2005, 15: 1609–1620.
Sauseng P, Klimesch W, Stadler W, Schabus M, Doppelmayr M, Hanslmayr S, et al. A shift of visual spatial attention is selectively associated with human EEG alpha activity. Eur J Neurosci 2005, 22: 2917–2926.
Johnson JA, Strafella AP, Zatorre RJ. The role of the dorsolateral prefrontal cortex in bimodal divided attention: Two transcranial magnetic stimulation studies. J Cogn Neurosci 2007, 19: 907–920.
Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci 2009, 32: 209–224.
Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science 2009, 324: 1207–1210.
Roberts MJ, Lowet E, Brunet NM, Ter Wal M, Tiesinga P, Fries P, et al. Robust gamma coherence between macaque V1 and V2 by dynamic frequency matching. Neuron 2013, 78: 523–536.
Vinck M, Lima B, Womelsdorf T, Oostenveld R, Singer W, Neuenschwander S, et al. Gamma-phase shifting in awake monkey visual cortex. J Neurosci 2010, 30: 1250–1257.
Laureys S, Owen AM, Schiff ND. Brain function in coma, vegetative state, and related disorders. Lancet Neurol 2004, 3: 537–546.
Zhu YC, Jiang XX, Ji WD. The mechanism of cortico-striato-thalamo-cortical neurocircuitry in response inhibition and emotional responding in attention deficit hyperactivity disorder with comorbid disruptive behavior disorder. Neurosci Bull 2018, 34: 566–572.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2017YFA0105203), the Strategic Priority Research Program of the Chinese Academy of Sciences (CAS) (XDB32040200 and XDB32030200), the Key Research Program of Frontier Sciences, CAS (QYZDJ-SSW-SMC019), and the National Natural Science Foundation of China (81871398, U1636121, and 31571003).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Niu, W., Jiang, Y., Zhang, X. et al. Changes of Effective Connectivity in the Alpha Band Characterize Differential Processing of Audiovisual Information in Cross-Modal Selective Attention. Neurosci. Bull. 36, 1009–1022 (2020). https://doi.org/10.1007/s12264-020-00550-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-020-00550-2