Abstract
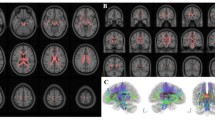
In this study, we aimed to (1) identify white matter (WM) deficits underlying the consciousness level in patients with disorders of consciousness (DOCs) using diffusion tensor imaging (DTI), and (2) evaluate the relationship between DTI metrics and clinical measures of the consciousness level in DOC patients. With a cohort of 8 comatose, 8 unresponsive wakefulness syndrome/vegetative state, and 14 minimally conscious state patients and 25 patient controls, we performed group comparisons of the DTI metrics in 48 core WM regions of interest (ROIs), and examined the clinical relevance using correlation analysis. We identified multiple abnormal WM ROIs in DOC patients compared with normal controls, and the DTI metrics in these ROIs were significantly correlated with clinical measures of the consciousness level. Therefore, our findings suggested that multiple WM tracts are involved in the impaired consciousness levels in DOC patients and demonstrated the clinical relevance of DTI for DOC patients.



Similar content being viewed by others
References
Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD. Detecting awareness in the vegetative state. Science 2006, 313: 1402.
Giacino JT, Fins JJ, Laureys S, Schiff ND. Disorders of consciousness after acquired brain injury: the state of the science. Nat Rev Neurol 2014, 10: 99–114.
Boly M, Tshibanda L, Vanhaudenhuyse A, Noirhomme Q, Schnakers C, Ledoux D, et al. Functional connectivity in the default network during resting state is preserved in a vegetative but not in a brain dead patient. Hum Brain Mapp 2009, 30: 2393–2400.
Boly M, Faymonville M-E, Schnakers C, Peigneux P, Lambermont B, Phillips C, et al. Perception of pain in the minimally conscious state with PET activation: an observational study. Lancet Neurol 2008, 7: 1013–1020.
Vanhaudenhuyse A, Noirhomme Q, Tshibanda LJ-F, Bruno M-A, Boveroux P, Schnakers C, et al. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain 2010, 133: 161–171.
Qin P, Wu X, Huang Z, Duncan NW, Tang W, Wolff A, et al. How are different neural networks related to consciousness? Ann Neurol 2015, 78: 594–605.
Wu X, Zou Q, Hu J, Tang W, Mao Y, Gao L, et al. Intrinsic functional connectivity patterns predict consciousness level and recovery outcome in acquired brain injury. J Neurosci 2015, 35: 12932–12946.
Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 1996, 111: 209–219.
Voss HU, Uluğ AM, Dyke JP, Watts R, Kobylarz EJ, McCandliss BD, et al. Possible axonal regrowth in late recovery from the minimally conscious state. J Clin Invest 2006, 116: 2005–2011.
Fernández-Espejo D, Soddu A, Cruse D, Palacios EM, Junque C, Vanhaudenhuyse A, et al. A role for the default mode network in the bases of disorders of consciousness. Ann Neurol 2012, 72: 335–343.
Fernández-Espejo D, Bekinschtein T, Monti MM, Pickard JD, Junque C, Coleman MR, et al. Diffusion weighted imaging distinguishes the vegetative state from the minimally conscious state. Neuroimage 2011, 54: 103–112.
Lant ND, Gonzalez-Lara LE, Owen AM, Fernández-Espejo D. Relationship between the anterior forebrain mesocircuit and the default mode network in the structural bases of disorders of consciousness. Neuroimage Clin 2015, 10: 27–35.
Newcombe VFJ, Williams GB, Scoffings D, Cross J, Carpenter TA, Pickard JD, et al. Aetiological differences in neuroanatomy of the vegetative state: insights from diffusion tensor imaging and functional implications. J Neurol Neurosurg Psychiatr 2010, 81: 552–561.
Posner JB, Plum F. Plum and Posner’s Diagnosis of Stupor and Coma. 4th ed. New York: Oxford University Press, 2007.
PVS TM-STF on. Medical aspects of the persistent vegetative state. New Engl J Med 1994, 330: 1572–1579.
Giacino JT, Ashwal S, Childs N, Cranford R, Jennett B, Katz DI, et al. The minimally conscious state: definition and diagnostic criteria. Neurology 2002, 58: 349–353.
Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2: 81–84.
Giacino JT, Kalmar K, Whyte J. The JFK coma recovery scale-revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil 2004, 85: 2020–2029.
Cui Z, Zhong S, Xu P, Gong G, He Y. PANDA: a pipeline toolbox for analyzing brain diffusion images. Front Hum Neurosci 2013, 7: 42.
Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage 2012, 62: 782–790.
Winklewski PJ, Sabisz A, Naumczyk P, Jodzio K, Szurowska E, Szarmach A. Understanding the physiopathology behind axial and radial diffusivity changes—What do we know? Front Neurol 2018, 9: 92.
Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 2008, 40: 570–582.
Faria AV, Zhang J, Oishi K, Li X, Jiang H, Akhter K, et al. Atlas-based analysis of neurodevelopment from infancy to adulthood using diffusion tensor imaging and applications for automated abnormality detection. Neuroimage 2010, 52: 415–428.
Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 2003, 20: 1714–1722.
Kier EL, Staib LH, Davis LM, Bronen RA. MR imaging of the temporal stem: anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer’s loop of the optic radiation. Am J Neuroradiol 2004, 25: 677–691.
Burzynska AZ, Jiao Y, Knecht AM, Fanning J, Awick EA, Chen T, et al. White matter integrity declined over 6-months, but dance intervention improved integrity of the fornix of older adults. Front Aging Neurosci 2017, 9: 59.
Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol 1949, 1: 455–473.
Northoff G. Unlocking the Brain: Volume 2: Consciousness. New York: Oxford University Press, 2014.
Parvizi J, Damasio AR. Neuroanatomical correlates of brainstem coma. Brain 2003, 126: 1524–1536.
Starzl TE, Taylor CW, Magoun HW. Ascending conduction in reticular activating system, with special reference to the diencephalon. J Neurophysiol 1951, 14: 461–477.
Delano-Wood L, Bangen KJ, Sorg SF, Clark AL, Schiehser DM, Luc N, et al. Brainstem white matter integrity is related to loss of consciousness and postconcussive symptomatology in veterans with chronic mild to moderate traumatic brain injury. Brain Imaging Behav 2015, 9: 500–512.
Monti MM, Vanhaudenhuyse A, Coleman MR, Boly M, Pickard JD, Tshibanda L, et al. Willful modulation of brain activity in disorders of consciousness. New Engl J Med 2010, 362: 579–589.
Laureys S, Schiff ND. Coma and consciousness: Paradigms (re)framed by neuroimaging. Neuroimage 2012, 61: 478–491.
Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WYI, Dai G, et al. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage 2008, 41: 1267–1277.
Dai JK, Wang SX, Shan D, Niu HC, Lei H. Super-resolution track-density imaging reveals fine anatomical features in tree shrew primary visual cortex and hippocampus. Neurosci Bull 2017, 1: 1–11.
Schnakers C, Vanhaudenhuyse A, Giacino J, Ventura M, Boly M, Majerus S, et al. Diagnostic accuracy of the vegetative and minimally conscious state: clinical consensus versus standardized neurobehavioral assessment. BMC Neurol 2009, 9: 35.
Acknowledgements
This work was supported by the Natural Science Foundation of China (81571025), International Cooperation Project from Shanghai Science Foundation (18410711300), the National Science Foundation for Distinguished Young Scholars of China (81025013), National Basic Research Development Program (973 Program) of China (2012CB720700, 2010CB945500, 2012CB966300, and 2009CB941100), the National Natural Science Foundation of China (81322021), the Beijing Nova Program (Z121110002512032), the Project for National 985 Engineering of China (985III-YFX0102), the “Dawn Tracking” Program of Shanghai Education Commission (10GG01), the Shanghai Natural Science Foundation (08411952000 and 10ZR1405400), the National Natural Science Young Foundation in China (81201033), the grants of Shanghai Health Bureau (20114358), the National High-Technology Development Project (863 Project) of China (2015AA020501), the Program for New Century Excellent Talents in University of China (NCET-10-0356), and the National Program for the Support of Top-Notch Young Professionals. Dr. Georg Northoff is supported by the Michael Smith Foundation, the CRC, and the CIHR. Jiaying Zhang is supported by the China Scholarship Council.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors claim that there are no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, X., Zhang, J., Cui, Z. et al. White Matter Deficits Underlying the Impaired Consciousness Level in Patients with Disorders of Consciousness. Neurosci. Bull. 34, 668–678 (2018). https://doi.org/10.1007/s12264-018-0253-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-018-0253-3