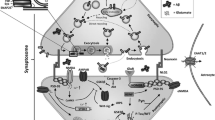
Abstract
Synapsins serve as flagships among the presynaptic proteins due to their abundance on synaptic vesicles and contribution to synaptic communication. Several studies have emphasized the importance of this multi-gene family of neuron-specific phosphoproteins in maintaining brain physiology. In the recent times, increasing evidence has established the relevance of alterations in synapsins as a major determinant in many neurological disorders. Here, we give a comprehensive description of the diverse roles of the synapsin family and the underlying molecular mechanisms that contribute to several neurological disorders. These physiologically important roles of synapsins associated with neurological disorders are just beginning to be understood. A detailed understanding of the diversified expression of synapsins may serve to strategize novel therapeutic approaches for these debilitating neurological disorders.



Similar content being viewed by others
References
Song SH, Augustine GJ. Synapsin isoforms and synaptic vesicle trafficking. Mol Cells 2015, 38: 936–940.
Fornasiero EF, Bonanomi D, Benfenati F, Valtorta F. The role of synapsins in neuronal development. Cell Mol Life Sci 2010, 67: 1383–1396.
Cesca F, Baldelli P, Valtorta F, Benfenati F. The synapsins: key actors of synapse function and plasticity. Prog Neurobiol 2010, 91: 313–348.
Masliah E, Terry R. The role of synaptic proteins in the pathogenesis of disorders of the central nervous system. Brain Pathol 1993, 3: 77–85.
Goelz SE, Nestler EJ, Chehrazi B, Greengard P. Distribution of protein I in mammalian brain as determined by a detergent-based radioimmunoassay. Proc Natl Acad Sci U S A 1981, 78: 2130–2134.
Hilfiker S, Pieribone VA, Czernik AJ, Kao H-T, Augustine GJ, Greengard P. Synapsins as regulators of neurotransmitter release. Philos Trans R Soc Lond B Biol Sci 1999, 354: 269–279.
Jovanovic JN, Benfenati F, Siow YL, Sihra TS, Sanghera JS, Pelech SL, et al. Neurotrophins stimulate phosphorylation of synapsin I by MAP kinase and regulate synapsin I-actin interactions. Proc Natl Acad Sci U S A 1996, 93: 3679–3683.
Zhang L, Zhao ZX. The impact of synapsins on synaptic plasticity and cognitive behaviors. Neurosci Bull 2006, 22:63–67.
Huttner, WB, Schiebler W, Greengard P, De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol 1983, 96: 1374–1388.
Hosaka M, Hammer RE, Südhof TC. A phospho-switch controls the dynamic association of synapsins with synaptic vesicles. Neuron 1999, 24: 377–387.
Sudhof TC, Czernik AJ, Kao HT, Takei K, Johnston PA, Horiuchi A, et al. Synapsins: mosaics of shared and individual domains in a family of synaptic vesicle phosphoproteins. Science 1989, 245: 1474–1480.
Kao HT, Porton B, Czernik AJ, Feng J, Yiu G, HÌring M, et al. A third member of the synapsin gene family. Proc Natl Acad Sci U S A 1998, 95: 4667–4672.
Hosaka M, Sudhof T. Synapsin III, a novel synapsin with an unusual regulation by Ca2+. J Biol Chem 1998, 273: 13371–13374.
Ferreira A, Rapoport M. The synapsins, beyond the regulation of neurotransmitter release. Cell Mol Life Sci 2002, 59: 589–595.
Coleman WL, Bykhovskaia M. Synapsin I accelerates the kinetics of neurotransmitter release in mouse motor terminals. Synapse 2009, 63: 531–533.
Hendricks BK, Shi R. Mechanisms of neuronal membrane sealing following mechanical trauma. Neurosci Bull 2014, 30: 627–44.
Ferreira A, Chin LS, Li L, Lanier LM, Kosik KS, Greengard P. Distinct roles of synapsin I and synapsin II during neuronal development. Mol Med 1998, 4: 22–28.
Terada S, Tsujimoto T, Takei Y, Takahashi T, Hirokawa N. Impairment of inhibitory synaptic transmission in mice lacking synapsin I. J Cell Biol 1999, 145: 1039–1048.
Li L, Chin LS, Shupliakov O, Brodin L, Sihra TS, Hvalby O, et al. Impairment of synaptic vesicle clustering and of synaptic transmission, and increased seizure propensity, in synapsin I-deficient mice. Proc Natl Acad Sci 1995, 92: 9235–9239.
Ferreira A, Kosik KS, Greengard P, Han HQ. Aberrant neurites and synaptic vesicle protein deficiency in synapsin II-depleted neurons. Science 1994, 264: 977–979.
Ferreira A, Han HQ, Greengard P, Kosik KS. Suppression of synapsin II inhibits the formation and maintenance of synapses in hippocampal culture. Proc Natl Acad Sci U S A 1995, 92: 9225–9229.
Coleman WL, Bill CA, Simsek-Duran F, Lonart G, Samigullin D, Bykhovskaia M. Synapsin II and calcium regulate vesicle docking and the cross-talk between vesicle pools at the mouse motor terminals. J Physiol 2008, 586: 4649–4673.
Gitler D, Cheng Q, Greengard P, Augustine GJ. Synapsin IIa controls the reserve pool of glutamatergic synaptic vesicles. J Neurosci 2008, 28: 10835–10843.
Han HQ, Nichols RA, Rubin MR, Bahler M, Greengard P. Induction of formation of presynaptic terminals in neuroblastoma cells by synapsin IIb. Nature 1991, 349: 697–700.
Ferreira A, Kao HT, Feng J, Rapoport M, Greengard P. Synapsin III: developmental expression, subcellular localization and role in axon formation. J Neurosci 2000, 20: 3736–3744.
Kao HT, Li P, Chao HM, Janoschka S, Pham K, Feng J, et al. Early involvement of Synapsin III in neural progenitor cell development in the adult hippocampus. J Comp Neurol 2008, 507: 1860–1870.
Feng J, Chi P, Blanpied TA, Xu YM, Magarinos AM, Ferreira A, et al. Regulation of neurotransmitter release by synapsin III. J Neurosci 2002, 22: 4372–4380.
Anand R, Gill KD, Mahdi AA. Therapeutics of Alzheimer’s disease: past, present and future. Neuropharmacology 2014, 76: 27–50.
Norfray JF, Provenzale JM. Alzheimer’s disease: neuropathologic findings and recent advances in imaging. AJR Am J Roentgenol 2004, 182: 3–13.
Bozoki AC, Korolev IO, Davis NC, Hoisington LA, Berger KL. Disruption of limbic white matter pathways in mild cognitive impairment and Alzheimer’s disease: a DTI/FDG-PET study. Hum Brain Mapp 2012, 33: 1792–1802.
Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: the challenge of the second century. Sci Transl Med 2011, 3: 77sr1.
Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol 1991, 30: 572–580.
Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 1992, 42: 631–639.
Song Q, Xiang-You H, Hao X, Jiang-Ning Z. Regional alteration of synapsin I in the hippocampal formation of Alzheimer’s disease patients. Acta Neuropathol 2004, 107: 209–215.
Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci 2000, 3: 323–329.
Lin L, Yang SS, Chu J, Wang L, Ning LN, Zhang T, Jiang Q, Tian Q, Wang JZ. Region-specific expression of tau, amyloid-β protein precursor, and synaptic proteins at physiological condition or under endoplasmic reticulum stress in rats. J Alzheimer’s Dis 2014, 41: 1149–1163.
Scheff SW, Price DA, Ansari MA, Roberts KN, Schmitt FA, Ikonomovic MD, et al. Synaptic change in the posterior cingulate gyrus in the progression of Alzheimer’s disease. J Alzheimer’s Dis 2015, 43:1073–1090.
Scheff SW, Price DA. Alzheimer’s disease-related synapse loss in the cingulate cortex. J Alzheimer’s Dis 2001; 3: 495–505.
Hartl D, Nebrich G, Klein O, Stephanowitz H, Krause E, Rohe M. SORLA regulates calpain-dependent degradation of synapsin. Alzheimers Dement 2016, 12: 952–963.
Haleem K, Lippa CF, Smith TW, Kowa H, Wu J, Iwatsubo T. Presenilin-1 C410Y Alzheimer disease plaques contain synaptic proteins. Am J Alzheimers Dis Other Dement 2007, 22: 137–144.
Li S, Yan Y, Jiao Y, Gao Z, Xia Y, Kong L, et al. Neuroprotective effect of osthole on neuron synapses in an Alzheimer’s disease cell model via upregulation of microRNA-9. J Mol Neurosci 2016, 60: 71–81.
Adler BL, Yarchoan M, Hwang HM, Louneva N, Blair JA, Palm R, Smith MA, Lee HG, Arnold SE, Casadesus G. Neuroprotective effects of the amylin analogue pramlintide on Alzheimer’s disease pathogenesis and cognition. Neurobiol Aging 2014, 35: 793–801.
Dawson GR, Seabrook GR, Zheng H, Smith DW, Graham S, O’Dowd G, et al. Age-related cognitive deficits, impaired long-term potentiation and reduction in synaptic marker density in mice lacking the beta-amyloid precursor protein. Neurosci 1999, 90: 1–13.
Ho L, Guo Y, Spielman L, Petrescu O, Haroutunian V, Purohit D, et al. Altered expression of a-type but not b-type synapsin isoform in the brain of patients at high risk for Alzheimer’s disease assessed by DNA microarray technique. Neurosci Lett 2001, 298: 191–194.
Suemaru S, Sato K, Morimoto K, Yamada N, Sato T, Kuroda S. Increment of synapsin I immunoreactivity in the hippocampus of the rat kindling model of epilepsy. Neuroreport 2000, 11: 1319–1322.
Carl ES. Epilepsy: a review of selected clinical syndromes and advances in basic science. J Cereb Blood Flow Metab 2006, 26: 983–1004.
Henry TR, Roman DD. Presurgical epilepsy localiztion with interictal cerebral dysfunction. Epilepsy Behav 2011, 20: 194–208.
Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005, 46: 470–472.
Etholm L, Heggelund P. Seizure elements and seizure element transitions during tonic–clonic seizure activity in the synapsin I/II double knockout mouse: a neuroethological description. Epilepsy Behav 2009, 14: 582–590.
Fassio A, Patry Le, Congia S, Onofri F, Piton A, Gauthier J, et al. SYN1 loss-of-function mutations in autism and partial epilepsy cause impaired synaptic function. Hum Mol Genet 2011, 20: 2297–2307.
Garcia CC, Blair HJ, Seager M, Coulthard A, Tennant S. Identification of a mutation in synapsin I, a synaptic vesicle protein, in a family with epilepsy. J Med Genet 2004, 41: 183–186.
Lignani G, Raimondi A, Ferrea E, Rocchi A, Paonessa F, Cesca F, et al. Epileptogenic Q555X SYN1 mutant triggers imbalances in release dynamics and short-term plasticity. Hum Mol Genet 2013, 22: 2186–2199.
Prasad DK, Shaheen U, Satyanarayana U, Prabha TS, Jyothy A, Munshi A. Association of GABRA6 1519 T > C (rs3219151) and Synapsin II (rs37733634) gene polymorphisms with the development of idiopathic generalized epilepsy. Epilepsy Res 2014, 108: 1267–1273.
Harrison PJ. Schizophrenia susceptibility genes and neurodevelopment. Biol Psychiatry 2007, 61: 1119–1120.
Li B, Cui LB, Xi YB, Friston KJ, Guo F, Wang HN, Zhang LC, Bai YH, Tan QR, Yin H, Lu H. Abnormal effective connectivity in the brain is involved in auditory verbal hallucinations in schizophrenia. Neurosci Bull 2017, 33: 281–291.
Calabresi P, Centonze D, Gubellini P, Marfia GA, Pisani A, Sancesario G, et al. Synaptic transmission in the striatum: from plasticity to neurodegeneration. Prog Neurobiol 2000, 61: 231–265.
Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain 1999, 122: 593–624.
Dyck BA, Beyaert MG, Ferro MA, Mishra RK. Medial prefrontal cortical synapsin II knock-down induces behavioral abnormalities in the rat: examining synapsin II in the pathophysiology of schizophrenia. Schizophr Res 2011, 130: 250–259.
Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron 2000, 28: 53–67.
Vawter M, Thatcher L, Usen N, Hyde T, Kleinman J, Freed W. Reduction of synapsin in the hippocampus of patients with bipolar disorder and schizophrenia. Mol Psychiatry 2002, 7: 571–578.
Tan ML, Dyck BA, Gabriele J, Daya RP, Thomas N, Sookram C, et al. Synapsin II gene expression in the dorsolateral prefrontal cortex of brain specimens from patients with schizophrenia and bipolar disorder: effect of lifetime intake of antipsychotic drugs. Pharmacogenomics J 2014, 14: 63–69.
Porton B, Wetsel WC. Reduction of synapsin III in the prefrontal cortex of individuals with schizophrenia. Schizophr Res 2011, 94: 366–370.
Saviouk V, Moreau MP, Tereshchenko IV, Brzustowicz LM. Association of synapsin 2 with schizophrenia in families of Northern European ancestry. Schizophr Res 2007, 96: 100–111.
Chen Q, He G, Wang XY, Chen QY, Liu XM, Gu ZZ, et al. Positive association between synapsin II and schizophrenia. Biol Psychiatry 2004, 56: 177–181.
Chen Q, He G, Qin W, Chen QY, Zhao XZ, Duan SW, et al. Family-based association study of synapsin II and schizophrenia. Am J Hum Genet 2004, 75: 873–877.
Gill M, Vallada H, Collier D, Sham P. A combined analysis of D22S278 marker alleles in affected sib-pairs: Support for a susceptibility locus for schizophrenia at chromosome 22q12. Am J Med Genet 1996, 67: 40–45.
Vallada H, Curtis D, Sham P, Kunugi H, Zhao JH, Murray R, et al. A transmission disequilibrium and linkage analysis of D22S278 marker alleles in 574 families: further support for a susceptibility locus for schizophrenia at 22q12. Schizophr Res 1998, 32: 115–121.
Porton B, Wetsel WC, Kao HT. Synapsin III: role in neuronal plasticity and disease. Semin Cell Dev Biol 2007, 22: 416–424.
Porton B, Ferreira A, DeLisi LE, Kao HT. A rare polymorphism affects a mitogen-activated protein kinase site in synapsin III: possible relationship to schizophrenia. Biol Psychiatry 2004, 55: 118–125.
Lachman HM, Stopkova P, Papolos DF, Pedrosa E, Margolis B, Aghalar MR, et al. Analysis of synapsin III–196 promoter mutation in schizophrenia and bipolar disorder. Neuropsychobiol 2006, 53: 57–62.
Chen Q, Che R, Wang X, O’Neill FA, Walsh D, Tang W, et al. Association and expression study of synapsin III and schizophrenia. Neurosci Lett 2009, 465: 248–251.
Ohtsuki T, Ichiki R, Toru M, Arinami T. Mutational analysis of the synapsin III gene on chromosome 22q12-q13 in schizophrenia. Psychiatry Res 2000, 94: 1–7.
Tsai MT, Hung CC, Tsai CY, Liu MY, Su YC, Chen YH, et al. Mutation analysis of synapsin III gene in schizophrenia. Am J Med Genet 2002, 114: 79–83.
Stöber G, Meyer J, Nanda I, Wienker TF, Saar K, Knapp M, et al. Linkage and family‐based association study of schizophrenia and the synapsin III locus that maps to chromosome 22q13. Am J Med Genet 2000, 96: 392–397.
Murphy BC, O’Reilly RL, Singh SM. DNA methylation and mRNA expression of SYN III, a candidate gene for schizophrenia. BMC Med Genet 2008, 9: 115.
Cruceanu C, Alda M, Nagy C, Freemantle E, Rouleau GA, Turecki G. H3K4 tri-methylation in synapsin genes leads to different expression patterns in bipolar disorder and major depression. Int J Neuropsychopharmacol 2013, 16: 289–299.
Cruceanu C, Kutsarova E, Chen ES, Checknita DR, Nagy C, Lopez JP, et al. DNA hypomethylation of Synapsin II CpG islands associates with increased gene expression in bipolar disorder and major depression. BMC Psychiatry 2016, 16: 286.
Cruceanu C, Alda M, Grof P, Rouleau GA, Turecki G. Synapsin II is involved in the molecular pathway of lithium treatment in bipolar disorder. PLoS One 2012, 7: e32680.
Nicot A, Ratnakar PV, Ron Y, Chen CC, Elkabes S. Regulation of gene expression in experimental autoimmune encephalomyelitis indicates early neuronal dysfunction. Brain 2003, 126: 398–412.
D’Alfonso S, Nistico L, Zavattari P, Marrosu MG, Murru R, Lai M, et al. Linkage analysis of multiple sclerosis with candidate region markers in Sardinian and Continental Italian families. Eur J Hum Genet 1999, 7: 177–185.
Liguori M1, Cittadella R, Manna I, Valentino P, La Russa A, Serra P, et al. Association between Synapsin III gene promoter polymorphisms and multiple sclerosis. J Neurol 2004, 251: 165–170.
Pette M, Pette DF, Muraro PA, Farnon E, Martin R, McFarland HF. Interferon-beta interferes with the proliferation but not with the cytokine secretion of myelin basic protein-specific, T-helper type 1 lymphocytes. Neurology 1997, 49: 385–392.
Weber F, Polak T, Gunther A, Kubuschok B, Janovskaja J, Bitsch A, et al. Synergistic immunomodulatory effects of interferon-β1b and the phosphodiesterase inhibitor pentoxifylline in patients with relapsing remitting multiple sclerosis. Ann Neurol 1998, 44: 27–34.
Rieckmann P, Albrecht M, Kitze B, Weber T, Tumani H, Broocks A, et al. Tumor necrosis factor-alpha messenger RNA expression in patients with relapsing-remitting multiple sclerosis is associated with disease activity. Ann Neurol 1995, 37: 82–88.
Polak T, Schlaf G, Scholl U, Krome-Cesar C, Mader M, Felgenhauer K, et al. Characterization of the human T cell response against the neuronal protein synapsin in patients with multiple sclerosis. J Neuroimmunol 2001, 115: 176–181.
Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci 2007, 30:575–621.
Vonsattel JP, DiFiglia M. Huntington disease. J Neuropathol Exp Neurol 1998, 57:369–384.
Liévens JC, Woodman B, Mahal A, Bates GP. Abnormal phosphorylation of synapsin I predicts a neuronal transmission impairment in the R6/2 Huntington’s disease transgenic mice. Mol Cell Neurosci 2002, 20: 638–648.
Xu Q, Huang S, Song M, Wang CE, Yan S, Liu X, et al. Synaptic mutant huntingtin inhibits synapsin-1 phosphorylation and causes neurological symptoms. J Cell Biol 2013, 202: 1123–1138.
Tang LT, Craig TJ, Henley JM. SUMOylation of synapsin Ia maintains synaptic vesicle availability and is reduced in an autism mutation. Nat Commun 2015, 6: 7728.
Schmidtko A, Del Turco D, Costea O, Ehnerta C, Niederbergera E. Essential role of the synaptic vesicle protein synapsin II in formalin-induced hyperalgesia and glutamate release in the spinal cord. Pain 2005, 115: 171–181.
Ikemoto A, Nakamura S, Akiguchi I, Hirano A. Differential expression between synaptic vesicle proteins and presynaptic plasma membrane proteins in the anterior horn of amyotrophic lateral sclerosis. Acta Neuropathol 2002, 103: 179–187.
Lukas TJ, Luo WW, Mao H, Cole N, Siddique T. Informatics-assisted protein profiling in a transgenic mouse model of amyotrophic lateral sclerosis. Mol Cell Proteomics 2006, 5: 1233–1244.
Masi A, DeMayo MM, Glozier N, Guastella AJ. An overview of autism spectrum disorder, heterogeneity and treatment options. Neurosci Bull 2017, 33: 183.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Rights and permissions
About this article
Cite this article
Mirza, F.J., Zahid, S. The Role of Synapsins in Neurological Disorders. Neurosci. Bull. 34, 349–358 (2018). https://doi.org/10.1007/s12264-017-0201-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-017-0201-7