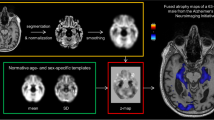
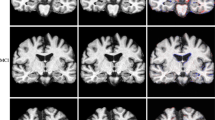
Abstract
Statistical analysis in neuroimaging (referred to as “neurostatistical imaging”) is important in clinical neurology. Here, neurostatistical imaging and its superiority for diagnosing dementia are reviewed. In neurodegenerative dementia, the proportional distribution of brain perfusion, metabolism, or atrophy is important for understanding the symptoms and status of patients and for identifying regions of pathological damage. Although absolute quantitative changes are important in vascular disease, they are less important than relative values in neurodegenerative dementia. Even under resting conditions in healthy individuals, the distribution of brain perfusion and metabolism is asymmetrical and differs among areas. To detect small changes, statistical analysis such as the Z-score — the number of standard deviations by which a patient’s voxel value differs from the normal mean value — comparing normal controls is useful and also facilitates clinical assessment. Our recent finding of a longitudinal one-year reduction of glucose metabolism around the olfactory tract in Alzheimer’s disease using the recently-developed DARTEL normalization procedure is also presented. Furthermore, a newly-developed procedure to assess brain atrophy with CT-based voxel-based morphometry is illustrated. The promising possibilities of CT in neurostatistical imaging are also presented.
Similar content being viewed by others
References
Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med 1995, 36: 1238–1248.
Kobayashi S, Tateno M, Utsumi K, Takahashi A, Morii H, Saito T. Two-layer appearance on brain perfusion SPECT in idiopathic normal pressure hydrocephalus: a qualitative analysis by using easy Z-score imaging system, eZIS. Dement Geriatr Cogn Disord 2009, 28: 330–337.
Minoshima S, Foster NL, Kuhl DE. Posterior cingulate cortex in Alzheimer’s disease. Lancet 1994, 344: 895.
Ohnishi T, Matsuda H, Tabira T, Asada T, Uno M. Changes in brain morphology in Alzheimer disease and normal aging: is Alzheimer disease an exaggerated aging process? AJNR Am J Neuroradiol 2001, 22: 1680–1685.
Klunk WE, Engler H, Nordberg A, Wang YM, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol 2004, 55: 306–319.
Maruyama M, Shimada H, Suhara T, Shinotoh H, Ji B, Maeda J, et al. Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron 2013, 79: 1094–1108.
Ashburner J, Friston KJ. Voxel-based morphometry — The methods. Neuroimage 2000, 11: 805–821.
Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol 1997, 42: 85–94.
Kogure D, Matsuda H, Ohnishi T, Asada T, Uno M, Kunihiro T, et al. Longitudinal evaluation of early Alzheimer’s disease using brain perfusion SPECT. J Nucl Med 2000, 41: 1155–1162.
Hirata Y, Matsuda H, Nemoto K, Ohnishi T, Hirao K, Yamashita F, et al. Voxel-based morphometry to discriminate early Alzheimer’s disease from controls. Neurosci Lett 2005, 382: 269–274.
Imabayashi E, Matsuda H, Asada T, Ohnishi T, Sakamoto S, Nakano S, et al. Superiority of 3-dimensional stereotactic surface projection analysis over visual inspection in discrimination of patients with very early Alzheimer’s disease from controls using brain perfusion SPECT. J Nucl Med 2004, 45: 1450–1457.
Kawachi T, Ishii K, Sakamoto S, Sasaki M, Mori T, Yamashita F, et al. Comparison of the diagnostic performance of FDG-PET and VBM-MRI in very mild Alzheimer’s disease. Eur J Nucl Med Mol Imaging 2006, 33: 801–809.
Matsuda H, Mizumura S, Nagao T, Ota T, Iizuka T, Nemoto K, et al. Automated discrimination between very early Alzheimer disease and controls using an easy Z-score imaging system for multicenter brain perfusion single-photon emission tomography. Am J Neuroradiol 2007, 28: 731–736.
Mito Y, Yoshida K, Yabe I, Makino K, Hirotani M, Tashiro K, et al. Brain 3D-SSP SPECT analysis in dementia with Lewy bodies, Parkinson’s disease with and without dementia, and Alzheimer’s disease. Clin Neurol Neurosurg 2005, 107: 396–403.
Lim SM, Katsifis A, Villemagne VL, Best R, Jones G, Saling M, et al. The 18F-FDG PET cingulate island sign and comparison to 123I-β-CIT SPECT for diagnosis of dementia with Lewy bodies. J Nucl Med 2009, 50: 1638–1645.
Nakatsuka T, Imabayashi E, Matsuda H, Sakakibara R, Inaoka T, Terada H. Discrimination of dementia with Lewy bodies from Alzheimer’s disease using voxel-based morphometry of white matter by statistical parametric mapping 8 plus diffeomorphic anatomic registration through exponentiated Lie algebra. Neuroradiology 2013, 55: 559–566.
Sakurai K, Imabayashi E, Tokumaru AM, Hasebe S, Murayama S, Morimoto S, et al. The feasibility of white matter volume reduction analysis using SPM8 plus DARTEL for the diagnosis of patients with clinically diagnosed corticobasal syndrome and Richardson’s syndrome. Neuroimage Clin 2014. DOI: 10.1016/j.nicl.2014.02.009
Villain N, Fouquet M, Baron JC, Mezenge F, Landeau B, de La Sayette V, et al. Sequential relationships between grey matter and white matter atrophy and brain metabolic abnormalities in early Alzheimer’s disease. Brain 2010, 133: 3301–3314.
Fouquet M, De sgranges B, Landeau B, Duchesnay E, Mezenge F, de la Sayette V, et al. Longitudinal brain metabolic changes from amnestic mild cognitive impairment to Alzheimer’s disease. Brain 2009, 132: 2058–2067.
Imabayashi E, Matsuda H, Yoshimaru K, Kuji I, Seto A, Shimano Y, et al. Pilot data on telmisartan short-term effects on glucose metabolism in the olfactory tract in Alzheimer’s disease. Brain Behav 2011, 1: 63–69.
Imabayashi E, Matsuda H, Tabira T, Arima K, Araki N, Ishii K, et al. Comparison between brain CT and MRI for voxel-based morphometry of Alzheimer’s disease. Brain Behav 2013, 3: 487–493.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Imabayashi, E., Inoue, T. Neurostatistical imaging for diagnosing dementia: translational approach from laboratory neuroscience to clinical routine. Neurosci. Bull. 30, 755–764 (2014). https://doi.org/10.1007/s12264-014-1464-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-014-1464-x