Abstract
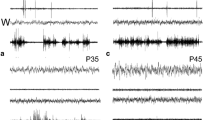
A comprehensive overview is presented of the literature dealing with the development of sleep-like motility and neuronal activity patterns in non-vertebrate animals. it has been established that spontaneous, periodically modulated, neurogenic bursts of movement appear to be a universal feature of prenatal behavior. New empirical data are presented showing that such’ seismic sleep’ or ‘rapid-body-movement’ bursts in cuttlefish persist for some time after birth. Extensive ontogenetic research in both vertebrates and non-vertebrates is thus essential before current hypotheses about the phylogeny of motorically active sleep-like states can be taken seriously.
Similar content being viewed by others
References
Corner M, van der Togt C. No phylogeny without ontogeny: a comparative and developmental search for the sources of sleep-like neural and behavioral rhythms. Neurosci Bull 2012, 28: 25–38.
Fuller PM, Saper CB, Lu J. The pontine REM switch: past and present. J Physiol 2007, 584: 735–741.
Jouvet M. Paradoxical Sleep—a Study of its Nature and Mechanisms. Prog Brain Res 1965, 18: 20–62.
Jouvet M. The Paradox of Sleep/ Le Sommeil et le Rêve. Cambridge, MA: MiT Press, 1999.
Siegel JM. Functional implications of sleep development. PLoS Biol 2005, 3: e178.
Valatx JL. The ontogeny and physiology confirms the dual nature of sleep states. Arch Ital Biol 2004, 142: 569–580.
Siegel JM. Do all animals sleep? Trends Neurosci 2008, 31: 208–213.
Lee Kavanau J. REM and NREM sleep as natural accompaniments of the evolution of warm-bloodedness. Neurosci Biobehav Rev 2002, 26: 889–906.
Lesku JA, Martinez-Gonzalez D, Rattenborg NC. Sleep and sleep states: phylogeny and ontogeny. in: Squire LR (ed.), Encyclopedia of Neuroscience. oxford: Academic Press, 2009: 963–971
Corner M. Spontaneous motor rhythms in early life3—phenomenological and neurophysiological aspects. Prog Brain Res 1978, 48: 349–366.
Corner MA. Sleep and the beginnings of behavior in the animal kingdom—studies of ultradian motility cycles in early life. Prog Neurobiol 1977, 8: 279–295.
Jouvet M. Le Chateau des Songes (The Castle of Dreams). Paris: Odile Jacob, 1992.
Raizen DM, Zimmerman JE, Maycock MH, Ta UD, You YJ, Sundaram MV, et al. Lethargus is a Caenorhabditis elegans sleep-like state. Nature 2008, 451: 569–572.
Mahowald MW, Cramer Bornemann MA, Schenck CH. State dissociation, human behavior, and consciousness. Curr Top Med Chem 2011, 11: 2392–2402.
French KA, Chang J, Reynolds S, Gonzalez R, Kristan WB 3rd, Kristan WB Jr. Development of swimming in the medicinal leech, the gradual acquisition of a behavior. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 2005, 191: 813–821.
Reynolds SA, French KA, Baader A, Kristan WB Jr. Development of spontaneous and evoked behaviors in the medicinal leech. J Comp Neurol 1998, 402: 168–180.
Zega G, Thorndyke MC, Brown ER. Development of swimming behaviour in the larva of the ascidian Ciona intestinalis. J Exp Biol 2006, 209: 3405–3412.
Ohmori H, Sasaki S. Development of neuromuscular transmission in a larval tunicate. J Physiol 1977, 269: 221–254.
Corner M. Rhythmicity in the Early Swimming of Anuran Larvae. J Embryol Exp Morphol 1964, 12: 665–671.
Fenelon V, Le Feuvre Y, Bem T, Meyrand P. Maturation of rhythmic neural network: role of central modulatory inputs. J Physiol Paris 2003, 97: 59–68.
Rehm KJ, Deeg KE, Marder E. Developmental regulation of neuromodulator function in the stomatogastric ganglion of the lobster, Homarus americanus. J Neurosci 2008, 28: 9828–9839.
Richards KS, Miller WL, Marder E. Maturation of lobster stomatogastric ganglion rhythmic activity. J Neurophysiol 1999, 82: 2006–2009.
O’Donovan MJ. The origin of spontaneous activity in developing networks of the vertebrate nervous system. Curr Opin Neurobiol 1999, 9: 94–104.
Swanson LW. Quest for the basic plan of nervous system circuitry. Brain Res Rev 2007, 55: 356–372.
Pereanu W, Spindler S, Im E, Buu N, Hartenstein V. The emergence of patterned movement during late embryogenesis of Drosophila. Dev Neurobiol 2007, 67: 1669–1685.
Crisp S, Evers JF, Fiala A, Bate M. The development of motor coordination in Drosophila embryos. Development 2008, 135: 3707–3717.
van Swinderen B, Nitz DA, Greenspan RJ. Uncoupling of brain activity from movement defines arousal states in Drosophila. Curr Biol 2004, 14: 81–87.
Eban-Rothschild AD, Bloch G. Differences in the sleep architecture of forager and young honeybees (Apis mellifera). J Exp Biol 2008, 211: 2408–2416.
BBC Natural History Unit. Aliens from inner space. Wild Film History 1983 [video].
Boletsky Sv, Boletsky MVv. Observations on the embryonic and early post-embryonic development of Rossia macrosoma (Mollusca, Cephalopoda). Helgolaender Wiss Meeresuntersuch 1973, 25: 135–161.
Tranter DJ, Augustine O. Observations on the life history of the blue-ringed octopus, Hapalochlaena maculosa. Marine Biol 1973, 18: 115–128.
von Boletzky S. Biology of early life stages in cephalopod molluscs. Adv Mar Biol 2003, 44: 143–203.
Meisel DV, Byrne RA, Mather JA, Kuba M. Behavioral sleep in octopus vulgaris. Vie et Milieu 2011, 61: 185–190.
Brown ER, Piscopo S, De Stefano R, Giuditta A. Brain and behavioural evidence for rest-activity cycles in octopus vulgaris. Behav Brain Res 2006, 172: 355–359.
Frank MG, Waldrop RH, Dumoulin M, Aton S, Boal JG. A preliminary analysis of sleep-like states in the cuttlefish Sepia officinalis. PLoS One 2012, 7: e38125.
Hanlon RT, Messenger JB. Adaptive coloration in young cuttlefish (Sepia offcinalis L.): the morphology and development of body patterns and their relation to behaviour. Phil Trans Roy Soc B (London) 1987, 320: 437–487.
Siegel JM, Manger PR, Nienhuis R, Fahringer HM, Pettigrew JD. Monotremes and the evolution of rapid eye movement sleep. Philos Trans R Soc Lond B Biol Sci 1998, 353: 1147–1157.
Koizumi O, Mizumoto H, Sugiyama T, Bode HR. Nerve net formation in the primitive nervous system of Hydra—an overview. Neurosci Res Suppl 1990, 13: S165–170.
Tabak J, O’Donovan MJ, Rinzel J. Differential control of active and silent phases in relaxation models of neuronal rhythms. J Comput Neurosci 2006, 21: 307–328.
Corner MA. Reciprocity of structure-function relations in developing neural networks: the odyssey of a self-organizing brain through research fads, fallacies and prospects. Prog Brain Res 1994, 102: 3–31.
Bosman L, Lodder JC, van ooyen A, Brussaard AB. Role of synaptic inhibition in spatiotemporal patterning of cortical activity. Prog Brain Res 2005, 147: 201–204.
Weiss PA. Deplantation of fragments of the nervous system in amphibians: central reorganization and the formation of nerves. J Exp Zool 1950, 113: 397–461.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Corner, M.A. Call it sleep — what animals without backbones can tell us about the phylogeny of intrinsically generated neuromotor rhythms during early development. Neurosci. Bull. 29, 373–380 (2013). https://doi.org/10.1007/s12264-013-1313-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-013-1313-3