Abstract
The new coronavirus (COVID-19) infection, first detected in Wuhan, China in 2019 has become a pandemic that has spread to nearly every country in the world. Through October 11, 2021, more than 23 billion confirmed cases and 4.8 million fatalities were reported globally. The bulk of individuals afflicted in India during the first wave were elderly persons. The second wave, however, resulted in more severe diseases and mortality in even younger age groups due to mutations in the wild virus. Symptoms may range from being asymptomatic to fatal acute respiratory distress syndrome (ARDS). In addition to respiratory symptoms, patients may present with gastrointestinal symptoms such as stomach pain, vomiting, loose stools, or mesenteric vein thrombosis. The frequency of patients presenting with thromboembolic symptoms has recently increased. According to certain studies, the prevalence of venous thromboembolism among hospitalized patients ranges from 9 to 25%. It was also shown that the incidence is significantly greater among critically sick patients, with a prevalence of 21–31%. Although the exact origin of thromboembolism is unknown, it is considered to be produced by several altered pathways that manifest as pulmonary embolism, myocardial infarction, stroke, limb gangrene, and acute mesenteric ischemia. Acute mesenteric ischemia (AMI) is becoming an increasingly prevalent cause of acute surgical abdomen in both intensive care unit (ICU) and emergency room (ER) patients. Mesenteric ischemia should be evaluated in situations with unexplained stomach discomfort. In suspected situations, appropriate imaging techniques and early intervention, either non-surgical or surgical, are necessary to avert mortality. The purpose of this article is to look at the data on acute mesenteric ischemia in people infected with COVID-19.
Similar content being viewed by others
Introduction
Aside from the respiratory system, the gastrointestinal system is the most common site of SARS-COV-2 infection. This might be because enterocyte and vascular endothelial membranes have large amounts of angiotensin-converting enzyme receptor 2, a membrane integral protein. As a result, the COVID virus induces direct enterocyte invasion as well as indirect endothelial injury-induced thrombosis/intestinal ischemia in the bowel [1]. ICU patients are more prone than non-ICU patients to suffer acute mesenteric ischemia. This might be because, in addition to the direct viral activity on vascular endothelium, ICU patients have extra persistent pro-inflammatory effects. Cases have been observed even among individuals who have recovered from infection [2]. A rising number of cases of acute mesenteric ischemia in COVID-19 patients have been reported in the literature since the outbreak of this pandemic (list of reported cases are summarized in the Table 1). AMI risk was shown to be increased with age, male sex, and comorbidities such as hypertension, obesity, and diabetes mellitus. Because of delayed clinical manifestation, AMI-related mortality is quite significant, with 60–80% [3].
Case summary
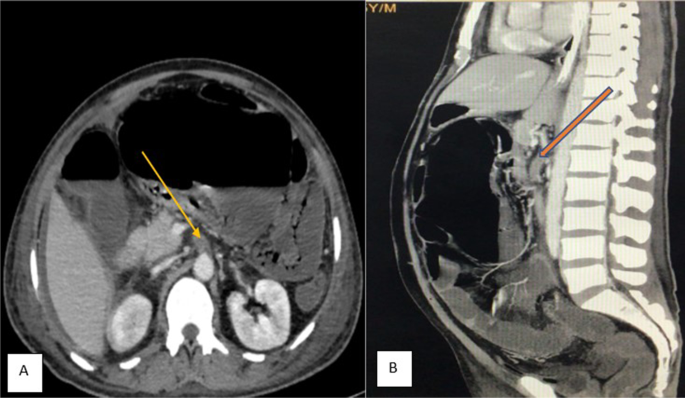
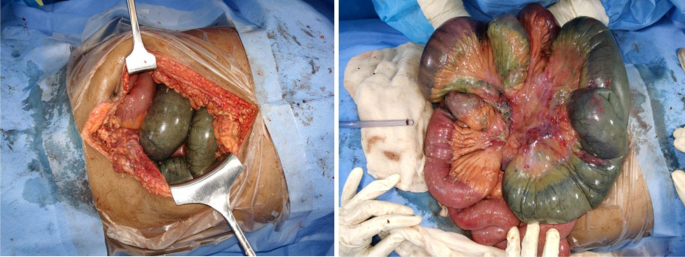
A 55 years old man with no known comorbidity presented to the emergency department of our institute with severe pain abdomen and multiple episodes of vomiting. He reported the recent recovery from the non-complicated COVID-related illness. He did not report any intake of anticoagulants. On clinical examination, abdomen was unremarkable. X-ray chest, x-ray erect abdomen, and ultrasound abdomen were unremarkable. Mesenteric ischemia was suspected and the patient was subjected to CT angiography abdomen, which revealed thrombus at the origin of the superior mesenteric artery and impending gangrene of the small bowel (Fig. 1). Emergency laparotomy was done and intraoperatively found the gangrenous bowel involving the distal jejunum and almost the entire ileum sparing the terminal ileum (Fig. 2). Resection of the gangrenous small bowel and end jejunostomy was done. Later, he was given ICU care, but unfortunately, the patient succumbed to multi-organ dysfunction syndrome.
Pathophysiology
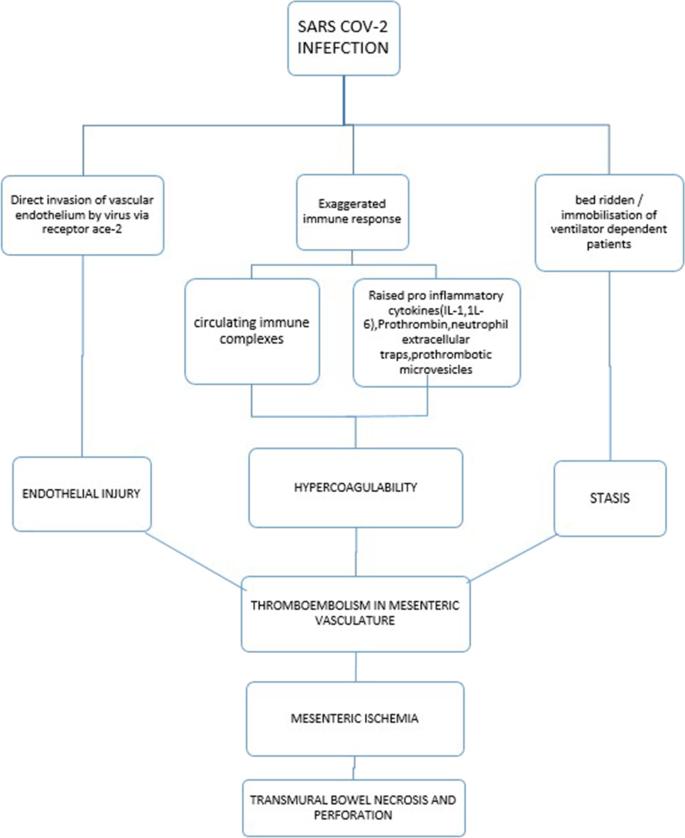
Although the specific etiology of hypercoagulable state and subsequent mesenteric ischemia in COVID-19 patients is unknown, these thromboembolic events can be related to alterations in all three Virchow triad characteristics (vascular endothelial injury, hypercoagulability, and stasis). A variety of variables complicate the etiology of thrombus development, one of which is vascular endothelial injury. Capillary permeability, hemostasis, and fibrinolysis are all maintained by the vascular endothelium (Fig. 3). Direct invasion causes endothelial cells to be damaged and lysed, resulting in an imbalance between pro and anticoagulant states [4]. Furthermore, vascular endothelial cells displayed morphological changes such as cellular expansion, retraction, and intercellular connection breakage [5]. The elevated levels of pro-inflammatory markers, von Willebrand factor, tissue factor, fibrinogen, and circulating microvesicles in the COVID-19 patients explain their hypercoagulability [6]. Antiphospholipid antibodies are elevated in some situations [7]. Patients who are critically ill, on limited oxygen support, and mechanical breathing are less mobilized, which increases the risk of deep venous thrombosis [3].
These mesenteric vascular thromboses cause acute hypoxia in the intestinal wall, which stimulates the renin-angiotensin system, causing mesenteric vasospasm and an elevated risk of hypoxic injury. SARS-COV binds to ACE 2 receptors in intestinal cells, causing cell lysis [8]. As a result, both hypoxia and direct invasion can trigger intestinal cell death. The loss of this epithelial barrier function in the gut promotes increased contact with enteric bacteria/endotoxins and viral particle penetration into the circulation [5]. The hypoxia continues, resulting in transmural infarction, perforation, and peritonitis. In one example of mesenteric ischemia induced by invasive mucormycosis, the presence of fungal components in the mesenteric microcirculation was documented [2]. See the flow chart summarizing the pathophysiology of mesenteric ischemia in covid-19 infection.
Clinical Presentation
Patients with mesenteric ischemia may exhibit a range of symptoms, from nonspecific complaints to peritonitis-like symptoms. Most of the patients developed symptoms a few days after being discharged successfully with proper symptomatic inpatient care. Although the respiratory symptoms predominate mesenteric ischemia presents with nonspecific abdominal symptoms such as loose stools, abdominal pain, nausea, vomiting, abdominal distension, and bleeding per rectum may occur in addition to the usual clinical presentation with respiratory features [6]. When opposed to arterial thrombosis, venous thrombosis has a delayed onset of symptoms. At first, sudden onset pain in the abdomen may be the sole symptom, and it may develop after 5–14 days. Abdominal clinical examination is nonyielding in the majority of cases. Abdominal signs would not develop unless the bowel gangrene or bowel perforation with peritonitis occurs [9].
Investigations
Blood investigations
Despite extensive study on the subject of acute mesenteric ischemia, the associated biomarkers were shown to be neither sensitive nor selective [10]. Elevated lactic acid levels and fibrin degradation products like D-dimer have low specificity and remain elevated in severe COVID-19 without AMI. However, biomarkers associated with hypercoagulable conditions aid in the initiation of preventive treatment and, to a lesser extent, in the management of COVID-related thrombotic events. Increased biomarkers of inflammation and infection include leukopenia (due to corticosteroid usage) and other signs such as C-reactive protein, procalcitonin, and IL-6. D-dimer, ferritin, prothrombin time, and lactate dehydrogenase are additional significant markers. The severity of increased lactate dehydrogenase and ferritin levels is associated with high mortality[8].
Radiological imaging
In the emergency room, an X-ray of the abdomen and an ultrasound are helpful for early examinations. X-ray of the erect abdomen helps in initial assessment in cases presented with features of obstruction or perforation. Ultrasound in the early phase may show SMA occlusion and bowel spasm or ultrasound findings in the early stages of acute mesenteric ischemia may appear normal [11]. In the intermediate phase, USG is not useful because of the presence of a large amount of gas-filled intestinal loops. In the late phase, USG may reveal fluid-filled lumen, bowel wall thinning, evidence of extra-luminal fluid, decreased or absent peristalsis. Therefore, USG may be helpful in the diagnosis of advanced bowel obstruction, gangrene, and perforation with peritoneal collection [12]. Ultrasonography revealed some other important features with distended and sludge-filled gall bladder with bile stasis. Portal venous gas also can be detected on ultrasonography which can be better characterized with the help of computed tomography [13].
Computed tomography
The gold standard investigation is CT angiography. CT observations commonly encountered in acute mesenteric ischemia secondary to COVID-19 includes thrombus in the aorta/SMA/portal circulation, augmentation of the bowel wall, thickness of the bowel wall with distention(> 3 cm), edema, and stranding of the mesentery, pneumatosis intestinalis or portal venous gas suggesting bowel wall ischemia, and non-enhancing thick bowel wall seen in bowel infarction, bowel perforation secondary to bowel infarction may present discontinuity of bowel wall with localized air collection. One should remember that pneumatosis intestinalis may also occur due to mechanical ventilation. Pneumoperitoneum occurs when there is severe intestinal necrosis and perforation. There were additional reports of nonspecific features such as a dilated gut with a fluid-filled lumen, distended gallbladder with bile stasis, features of solid organ ischemia, and pancreatitis [14]. MRI, despite its accessibility, has drawbacks such as a longer acquisition time and lower resolution than CT angiography [12].
Management
A summary of cases of acute mesenteric ischemia has been tabulated (Table 1). Management of acute mesenteric ischemia in COVID-19 includes the following:
-
Supportive measures: Crystalloid rehydration and empirical antibacterial treatment should begin before angiography or any surgical resection. Comorbidity management, hemodynamic support in unstable patients, and electrolyte balance correction are all critical components of patient care [10].
-
Anticoagulation: There is insufficient data in 19 patients to warrant thromboprophylaxis. According to the Tang et al. study, low-dose heparin prophylaxis decreased thrombotic events and mortality in those with D-dimer levels over 3 mg/ml. Despite the increased risk of bleeding, mesenteric ischemia should be treated with intraoperative and postoperative anticoagulation [15].
-
Revascularisation: Revascularization with catheter-directed thrombolysis and thrombectomy by percutaneous/surgical intervention can be explored in instances where there is no indication of significant intestinal ischemia. Catheter-directed thrombolysis with unfractionated heparin and recombinant tissue plasminogen activators can accomplish this. Because of the increased risk of re-thrombosis, vascular clearance is not indicated in instances of superior mesenteric vein thrombus [15].
-
Resection of the gangrenous bowel: Depending on clinical suspicion, a CT angiography examination of mesenteric vasculature and bowel health can be performed, and an emergency exploration call should be placed. Intraoperatively, if the patient is normotensive, has no sepsis or peritonitis, and the remaining bowel viability is unquestionable, the gangrenous bowel is to be removed, and the remaining bowel can be considered for re-anastomosis. In unfavorable circumstances, a stoma should be created following gangrenous bowel resection [11]. The margin dissection in venous thrombosis should be broader than in arterial thrombosis. To assure the bowel's survivability, abdominal closure should be temporary, and a relook laparotomy should be done 48 h later. Histopathological examination of the resected intestine may indicate patchy or widespread necrotic changes from mucosa to transmural thickness. In the submucosal vasculature, fibrin-containing microthrombi with perivascular neutrophilic infiltration is observed.
-
Management of short bowel syndrome: The therapy varies depending on the length of colon left after excision of infarcted bowel caused by mesenteric ischemia.
-
Medical- In severe diarrhea, fluid and electrolyte loss must be replaced. TPN for feeding and histamine-2 receptor antagonists or PPIs for stomach acid secretion reduction. Loperamide and diphenoxylate are anti-motility medicines that delay small intestine transit whereas Octreotide reduces the volume of gastrointestinal secretions.
-
Non-transplant surgical therapy- Done to improve the absorption capacity of the remaining intestine by restoring intestinal continuity. Increased nutrient and fluid absorption is the goal. Segmental reversal of the small bowel, fabrication of small intestinal valves, and electrical pacing of the small bowel are all procedures used to delay intestinal transit. Longitudinal intestinal lengthening and tailoring technique (LILT) and serial transverse arthroplasty process are two intestinal lengthening procedures (STEP).
-
Intestinal transplantation- Life-threatening problems such as liver failure, thrombosis of major central veins, frequent episodes of severe dehydration, and catheter-related sepsis are reasons for intestinal transplantation [16].
Prognosis
Acute mesenteric ischemia has a poor prognosis, and life is reliant on prompt diagnosis and treatment. If detected within 24 h, the likelihood of survival is 50%, but it declines to 30% beyond that [17].In operated cases, COVID infection acts as an independent risk factor and is responsible for higher mortality [18].
Conclusion
SARS-COV-2 infection even though initially thought to be respiratory infection; later cases detected presenting with multisystem involvement. The presentation may vary from asymptomatic or mildly symptomatic to severe respiratory distress syndrome or thromboembolic phenomenon requiring ICU care. The exact mechanism of thromboembolism is not established. However, the increasing number of acute mesenteric ischemia is quite alarming. The treating physician should be overcautious in patients presenting with abdominal symptoms either currently affected or recovered from COVID-related illness. In high-risk patients, early start of prophylactic anticoagulation may be beneficial. Earlier intervention is known acute mesenteric ischemia cases with operative or minimally invasive procedures may give higher survival benefits. It mandates more research to determine the causes of thromboembolism, as well as preventive and therapeutic anticoagulation in these individuals.
References
Jin B, Singh R, Ha SE, Zogg H, Park PJ, Ro S (2021) Pathophysiological mechanisms underlying gastrointestinal symptoms in patients with COVID-19. World J Gastroenterol. Baishideng Publishing Group Co 27:2341–52
Jain M, Tyagi R, Tyagi R, Jain G (2021) Post-COVID-19 gastrointestinal invasive mucormycosis. Indian J Surg 22:1–3
Kerawala AA, Das B, Solangi A (2021) Mesenteric ischemia in COVID-19 patients: a review of current literature. World J Clin Cases 9(18):4700–4708
Kichloo A, Dettloff K, Aljadah M, Albosta M, Jamal S, Singh J et al (2020) COVID-19 and hypercoagulability: a review. Clin Appl Thromb 26
Parry AH, Wani AH, Yaseen M (2020) Acute mesenteric ischemia in severe Coronavirus-19 (COVID-19): possible mechanisms and diagnostic pathway. Acad Radiol 27(8):1190
Cheung S, Quiwa JC, Pillai A, Onwu C, Tharayil ZJ, Gupta R (2020) Superior mesenteric artery thrombosis and acute intestinal ischemia as a consequence of COVID-19 infection. Am J Case Rep 21:1–3
Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W et al (2020) Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 382(17):e38
Al Mahruqi G, Stephen E, Abdelhedy I, Al WK (2021) Our early experience with mesenteric ischemia in COVID-19 positive patients. Ann Vasc Surg 73:129–132
Karna ST, Panda R, Maurya AP, Kumari S (2020) Superior mesenteric artery thrombosis in COVID-19 Pneumonia: an underestimated diagnosis—first case report in Asia. Indian J Surg 82(6):1235–1237
Singh B, Kaur P (2021) COVID-19 and acute mesenteric ischemia: a review of literature. Hematol Transfus Cell Ther 43(1):112–116
Janež J, Klen J (2021) Multidisciplinary diagnostic and therapeutic approach to acute mesenteric ischaemia: a case report with literature review. SAGE Open Med Case Rep 9:2050313X2110048
Mc W (2010) Acute mesenteric ischemia: diagnostic approach and surgical treatment. Semin Vasc Surg 23(1):9–20
Bhayana R, Som A, Li MD, Carey DE, Anderson MA, Blake MA et al (2020) Abdominal imaging findings in COVID-19: Preliminary observations. Radiology 297(1):E207–E215
Keshavarz P, Rafiee F, Kavandi H, Goudarzi S, Heidari F, Gholamrezanezhad A (2021) Ischemic gastrointestinal complications of COVID-19: a systematic review on imaging presentation. Clin Imaging 73:86–95
Bergqvist D, Svensson PJ (2010) Treatment of mesenteric vein thrombosis. Semin Vasc Surg 23(1):65–68
Seetharam P, Rodrigues G (2011) Short bowel syndrome: a review of management options. Saudi J Gastroenterol 17(4):229–235
Krothapalli N, Jacob J (2021) A rare case of acute mesenteric ischemia in the setting of COVID-19 infection. Cureus 13(3):0–4
Haffner MR, Le HV, Saiz AM, Han G, Fine J, Wolinsky P et al (2021) Postoperative In-hospital morbidity and mortality of patients with COVID-19 infection compared with patients without COVID-19 infection. JAMA Netw Open 4(4):10–13
Ucpinar BA, Sahin C (2020) Superior mesenteric artery thrombosis in a patient with COVID-19: a unique presentation. J Coll Physicians Surg Pak 30(10):S112–S114
Khesrani LS, Chana k, Sadar FZ, Dahdouh A, Ladjadj Y, Bouguermouh D (2020) Intestinal ischemia secondary to Covid-19. J Pediatr Surg Case Rep 61:101604
Norsa L, Valle C, Morotti D, Bonaffini PA, Indriolo A, Sonzogni A (2020) Intestinal ischemia in the COVID-19 era. Dig Liver Dis 52(10):1090–1091
Rodriguez-Nakamura RM, Gonzalez-Calatayud M, Martinez Martinez AR (2020) Acute mesenteric thrombosis in two patients with COVID-19. Two cases report and literature review. Int J Surg Case Rep 76:409–14
VartanogluAktokmakyan T, Tokocin M, Meric S, Celebi F (2021) Is mesenteric ischemia in COVID-19 patients a surprise? Surg Innov 28(2):236–238
Levolger S, Bokkers RPH, Wille J, Kropman RHJ, de Vries JPPM (2020) Arterial thrombotic complications in COVID-19 patients. J Vasc Surg Cases Innov Tech 6(3):454–459
Thuluva SK, Zhu H, Tan MML, Gupta S, Yeong KY, Wah STC et al (2020) A 29-year-old male construction worker from india who presented with left-sided abdominal pain due to isolated superior mesenteric vein thrombosis associated with SARS-CoV-2 infection. Am J Case Rep 21:1–5
Lari E, Lari A, AlQinai S, Abdulrasoul M, AlSafran S, Ameer A et al (2020) Severe ischemic complications in Covid-19—a case series. Int J Surg Case Rep 75(June):131–135
Singh B, Mechineni A, Kaur P, Ajdir N, Maroules M, Shamoon F et al (2020) Acute intestinal ischemia in a patient with COVID-19 infection. Korean J Gastroenterol 76(3):164–166
De Roquetaillade C, Chousterman BG, Tomasoni D, Zeitouni M, Houdart E (2020) Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID- 19. The COVID-19 resource centre is hosted on Elsevier Connect , the company ’ s public news and information. (January)
Sehhat S, Talebzadeh H, Hakamifard A, Melali H, Shabib S, Rahmati A et al (2020) Acute mesenteric ischemia in a patient with COVID-19: a case report. Arch Iran Med 23(9):639–643
Beccara LA, Pacioni C, Ponton S, Francavilla S, Cuzzoli A (2020) Arterial mesenteric thrombosis as a complication of SARS-CoV-2 infection. Eur J Case Rep Intern Med 7(5).
Ignat M, Philouze G, Aussenac-belle L (2020) Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID- 19 . The COVID-19 resource centre is hosted on Elsevier Connect , the company ’ s public news and information. (Jan)
Farina D, Rondi P, Botturi E, Renzulli M, Borghesi A, Guelfi D et al (2021) Gastrointestinal: bowel ischemia in a suspected coronavirus disease (COVID-19) patient. J Gastroenterol Hepatol 36(1):41
Azouz E, Yang S, Monnier-Cholley L, Arrivé L (2020) Systemic arterial thrombosis and acute mesenteric ischemia in a patient with COVID-19. Intensive Care Med 46(7):1464–1465
Vulliamy P, Jacob S, Davenport RA (2020) Acute aorto-iliac and mesenteric arterial thromboses as presenting features of COVID-19. Br J Haematol 189(6):1053–1054
Bianco F, Ranieri AJ, Paterniti G, Pata F, Gallo G (2020) Acute intestinal ischemia in a patient with COVID-19. Tech Coloproctol 24(11):1217–1218
Filho A do C, Cunha B da S (2020) Case report – inferior mesenteric vein thrombosis and COVID-19. 2020060282
Mitchell JM, Rakheja D, Gopal P (2021) SARS-CoV-2-related hypercoagulable state leading to ischemic enteritis secondary to superior mesenteric artery thrombosis. Clin Gastroenterol Hepatol 19(11):e111
English W, Banerjee S (2020) Coagulopathy and mesenteric ischaemia in severe SARS-CoV-2 infection. ANZ J Surg 90(9):1826
de Barry O, Mekki A, Diffre C, Seror M, El Hajjam M, Carlier RY (2020) Arterial and venous abdominal thrombosis in a 79-year-old woman with COVID-19 pneumonia. Radiol Case Rep 15(7):1054–1057
Kraft M, Pellino G, Jofra M, Sorribas M, Solís-Peña A, Biondo S, Espín-Basany E (2021) Incidence, features, outcome and impact on health system of de-novo abdominal surgical diseases in patients admitted with COVID-19. Surg J R Coll Surg Edinb Irel 19:e53–e58
Besutti G, Bonacini R, Iotti V, Marini G, Riva N, Dolci G et al (2020) Abdominal visceral infarction in 3 patients with COVID-19. Emerg Infect Dis 26(8):1926–1928
Kielty J, Duggan WP, O’Dwyer M (2020) Extensive pneumatosis intestinalis and portal venous gas mimicking mesenteric ischaemia in a patient with SARS-CoV-2. Ann R Coll Surg Engl 102(6):E145–E147
Pang JHQ, Tang JH, Eugene-Fan B (2021) A peculiar case of small bowel stricture in a coronavirus disease 2019 patient with congenital adhesion band and superior mesenteric vein thrombosis. Ann Vasc Surg 70:286–289
Osilli D, Pavlovica J, Mane R, Ibrahim M, Bouhelal A, Jacob S (2020) Case reports: mild COVID-19 infection and acute arterial thrombosis. J Surg Case Rep (9):1–3
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gupta, A., Sharma, O., Srikanth, K. et al. Review of Mesenteric Ischemia in COVID-19 Patients. Indian J Surg 85 (Suppl 1), 313–321 (2023). https://doi.org/10.1007/s12262-022-03364-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-022-03364-w