Abstract
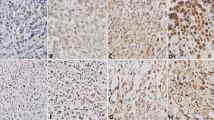
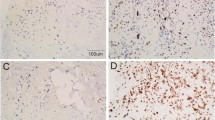
The aim of this study was to investigate the expression of DACH1 in osteosarcoma as well as its relationship with cell proliferation and angiogenesis in the tumor. DACH1 expression was detected by immunohistochemical staining in the serial sections of the osteosarcoma. The microvessel density (MVD) was counted by CD34 immunohistochemical staining, and immunohistochemical staining of PCNA staining showed the cell proliferation. The impacts of DACH1 expression on tumor proliferation and angiogenesis were evaluated by statistics. The DACH1 had different expression patterns in different osteosarcoma. Conventional osteosarcoma showed stronger DACH1 staining (conventional vs. parosteal: P = 0.037; conventional vs. periosteal: P = 0.028) and more PCNA-positive tumor cells than parosteal and periosteal osteosarcoma (conventional vs. parosteal: P = 0.041; conventional vs. periosteal: P = 0.045), the difference was significant. In addition, conventional osteosarcoma showed more cytoplasmic staining of DACH1 than parosteal and periosteal (conventional vs. parosteal: P = 0.023; conventional vs. periosteal: P = 0.030). Parosteal and periosteal osteosarcoma showed no significant difference in DACH1 expression and cell proliferation index. On the other hand, DACH1 different expression patterns showed significantly different impacts on angiogenesis. In spite of the different subtypes of osteosarcoma, the MVD showed a significant difference in cytoplasmic and nuclear expression patterns of DACH1 (nuclear expression vs. cytoplasmic expression: 5.72 ± 1.19 vs. 9.65 ± 1.24, P = 0.042). Moreover, in the conventional osteosarcoma, the MVD also showed a significant difference in DACH1 cytoplasmic and nuclear staining (nuclear expression vs. cytoplasmic expression: 5.58 ± 0.71 vs. 13.65 ± 1.30, P = 0.019). However, the DACH1 expression intensity showed no significant different impacts on MVD of all kinds of osteosarcoma. DACH1 had different expression patterns and intensity. Cytoplasmic and nuclear expression of DACH1 might play different roles in cell proliferation and angiogenesis of osteosarcoma. Cytoplasmic DACH1 might promote cell proliferation and be associated with angiogenesis.

Similar content being viewed by others
References
Mardon G, Solomon NM, Rubin GM (1994) Dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development 120:3473–3486
Chen R, Amouri M, Zhang Z, Mardon G (1997) Dachshund and eyes absent proteins form a complex and function synergistically to induce ectopic eye development in Drosophila. Cell 91:893–903
Shen W, Mardon G (1997) Ectopic eye development in drosophila induced by directed dachsund expression. Development 124:45–52
Wawersik S, Maas RL (2000) Vertebrate eye development as modeled in Drosophila. Hum Mol Genet 9:917–925
Bai J, Montell D (2002) Eyes absent, a key repressor of polar cell fate during Drosophila oogenesis. Development 129:5377–5388
Fabrizio JJ et al (2003) A somatic role for eyes absent (eya) and sine oculis (so) in Drosophila spermatocyte development. Dev Biol 258:117–128
Bonini NM et al (1998) Multiple roles of the eyes absent gene in Drosophila. Dev Biol 196:42–57
Popov VM, Wu K, Powell MJ, Mardon G, Wang C, Pestell RG (2010) The Dachshund gene in development and hormone-responsive tumorigenesis. Trends Endocrinol Metab 21(1):41
Sundaram K, Mani SK, Kitatani K, Wu K, Pestell RG, Reddy SV (2008) DACH1 negatively regulates the human RANK ligand gene expression in stromal/preosteoblast cells. J Cell Biochem 103(6):1747–1759
Wu K, Li A, Rao M, Liu M, Dailey V, Yang Y et al (2006) DACH1 is a cell fate determination factor that inhibits cyclin D1 and breast tumor growth. Mol Cell Biol 26:7116–7129
Popov VM, Zhou J, Shirley LA, Quong J, Yeow W-S, Wright JA, Wu K, Rui H, Vadlamudi RK, Jiang J, Kumar R, Wang C, Pestell RG (2009) The cell fate determination factor DACH1 is expressed in estrogen receptor-A–positive breast cancer and represses estrogen receptor-A signaling. Cancer Res 69(14):5752–5760
Sunde JS, Donninger H, Wu K et al (2006) Factor-b signaling in ovarian cancer that contribute to the inhibition of transforming growth expression profiling identifies altered expression of genes. Cancer Res 66:8404–8412
Nan F, Lü Q, Zhou J et al (2009) Altered expression of DACH1 and cyclin D1 in endometrial cancer. Cancer Biol Ther 8:1534–1539
Wu K, Katiyar S, Li A, Liu M, Ju X, Popov VM, Jiao X, Lisanti MP, Casola A, Pestell RG (2008) Dachshund inhibits oncogene-induced breast cancer cellular migration and invasion through suppression of interleukin-8. Proc Natl Acad Sci USA 105:6924–6929
Wu K, Katiyar S, Witkiewicz A, Li A, McCue P, Song L-N, Tian L, Jin M, Pestell RG (2009) The cell fate determination factor Dachshund inhibits androgen receptor signaling and prostate cancer cellular growth. Cancer Res 69(8):3347–3355
Miller SJ, Lan ZD, Hardiman A et al (2010) Inhibition of Eyes Absent Homolog 4 expression induces malignant peripheral nerve sheath tumor necrosis. Oncogene 29(3):368–379
Fletcher CDM, Unni KK, Mertens F (eds) (2002) World Health Organization classification of tumours. In: Pathology and Genetics of Tumours of Soft Tissue and Bone. IARC Press, Lyon
Liang Y, Fu D, Hu G (2011) Metadherin: An emerging key regulator of the malignant progression of multiple cancers. Thoracic Cancer 2(4):143–148
Zhou J, Liu Y, Zhang W, Popov VM, Wang M, Pattabiraman N, Suñé C, Cvekl A, Wu K, Jiang J, Wang C, Pestell RG (2010) Transcription elongation regulator 1 is a co-integrator of the cell fate determination factor Dachshund homolog 1. J Biol Chem 285(51):40342–40350
DeAngelis T, Wu K, Pestell R, Baserga R (2011) The type 1 insulin-like growth factor receptor and resistance to DACH1. Cell Cycle 10(12):1956–1959
Wu K, Jiao X, Li Z, Katiyar S, Casimiro MC, Yang W, Zhang Q, Willmarth NE, Chepelev I, Crosariol M, Wei Z, Hu J, Zhao K, Pestell RG (2011) Cell fate determination factor Dachshund reprograms breast cancer stem cell function. J Biol Chem 286(3):2132–2142
Watanabe A, Ogiwara H, Ehata S et al (2011) Homozygously deleted gene DACH1 regulates tumor-initiating activity of glioma cells. Proc Natl Acad Sci USA 108(30):12384–12389
Zhong X, Li L, Wang M et al (2011) A proteomic approach to elucidate the multiple targets of selenium-induced cell-growth inhibition in human lung cancer.Thoracic Cancer 2(4):164–178
Maniwa Y, Nishio W, Yoshimura M (2011) Application of hRad9 in lung cancer treatment as a molecular marker and a molecular target. Thoracic Cancer 2(1):7–15
Author information
Authors and Affiliations
Corresponding author
Additional information
Peng Ren and Ming-zhi Gong contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ren, P., Gong, Mz., Wang, Zy. et al. DACH1 Expresison in Osteosarcoma and Its Relationship with Proliferation and Angiogenesis. Indian J Surg 77, 200–205 (2015). https://doi.org/10.1007/s12262-012-0761-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-012-0761-8