Abstract
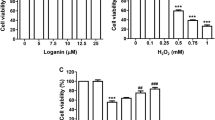
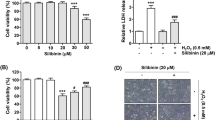
Previous studies have shown that honokiol, a bioactive compound originated from the Magnolia, has beneficial effects on various chronic diseases. Although our previous study has demonstrated that honokiol effectively protects C2C12 myoblasts against hydrogen peroxide (H2O2)-induced oxidative stress and cytotoxicity, the antioxidant mechanism in human retinal pigment epithelial (RPE) cells has not been reported yet. Therefore, this study investigates whether honokiol attenuate cytotoxicity of H2O2 to human RPE ARPE-19 cells. Our results showed that H2O2-induced loss of cell viability and increase of reactive oxygen species production in ARPE-19 cells were significantly alleviated by honokiol, which was associated with increased phosphorylation of nuclear factor erythroid 2-related factor 2 (Nrf2) and upregulation of heme oxygenase-1 (HO-1). In addition, honokiol markedly attenuated DNA damage and G2/M phase cell cycle arrest and improved apoptosis in H2O2-treated ARPE-19 cells. Furthermore, mitochondrial dysfunction by H2O2 was reversed by honokiol through preservation of mitochondrial membrane potential, decrease of Bax/Bcl-2 expression ratio, and inhibition of cytochrome c release into the cytoplasm. However, zinc protoporphyrin, an inhibitor of HO-1, significantly abrogated these preventive effects, suggesting a critical role of Nrf2/HO-1 in the antioxidant activity of honokiol. Taken together, these results demonstrate that activation of Nrf2/HO-1 antioxidant signaling by honokiol could rescue ARPE-19 cells from oxidative damage.
Similar content being viewed by others
References
Kim, J., Y. J. Lee, and J. Y. Won (2021) Molecular mechanisms of retinal pigment epithelium dysfunction in age-related macular degeneration. Int. J. Mol. Sci. 22: 12298.
Chichagova, V., D. Hallam, J. Collin, D. Zerti, B. Dorgau, M. Felemban, M. Lako, and D. H. Steel (2018) Cellular regeneration strategies for macular degeneration: past, present and future. Eye (Lond.) 32: 946–971.
Léveillard, T., N. J. Philp, and F. Sennlaub (2019) Is retinal metabolic dysfunction at the center of the pathogenesis of age-related macular degeneration? Int. J. Mol. Sci. 20: 762.
Sadda, S. R., R. Guymer, J. M. Monés, A. Tufail, and G. J. Jaffe (2020) Anti-vascular endothelial growth factor use and atrophy in neovascular age-related macular degeneration: systematic literature review and expert opinion. Ophthalmology 127: 648–659.
Wang, P., E. K. Chin, and D. Almeida (2021) Antioxidants for the treatment of retinal disease: summary of recent evidence. Clin. Ophthalmol. 15: 1621–1628.
Toma, C., S. De Cillà, A. Palumbo, D. P. Garhwal, and E. Grossini (2021) Oxidative and nitrosative stress in age-related macular degeneration: a review of their role in different stages of disease. Antioxidants (Basel) 10: 653.
Karakuş, M. M. and U. K. Çalışkan (2021) Phytotherapeutic and natural compound applications for age-related, inflammatory and serious eye ailments. Curr. Mol. Pharmacol. 14: 689–713.
Ikonne, E. U., V. O. Ikpeazu, and E. A. Ugbogu (2020) The potential health benefits of dietary natural plant products in age related eye diseases. Heliyon 6: e04408. (Erratum published 2021, Heliyon 7: e07069)
Rauf, A., A. Olatunde, M. Imran, F. A. Alhumaydhi, A. S. M. Aljohani, S. A. Khan, M. S. Uddin, S. Mitra, T. B. Emran, M. Khayrullin, M. Rebezov, M. A. Kamal, and M. A. Shariati (2021) Honokiol: a review of its pharmacological potential and therapeutic insights. Phytomedicine 90: 153647. (Erratum published 2021, Phytomedicine 92: 153769)
Ong, C. P., W. L. Lee, Y. Q. Tang, and W. H. Yap (2019) Honokiol: a review of its anticancer potential and mechanisms. Cancers (Basel) 12: 48.
Yuan, Y., X. Zhou, Y. Wang, Y. Wang, X. Teng, and S. Wang (2020) Cardiovascular modulating effects of magnolol and honokiol, two polyphenolic compounds from traditional Chinese Medicine-Magnolia officinalis. Curr. Drug Targets 21: 559–572.
Talarek, S., J. Listos, D. Barreca, E. Tellone, A. Sureda, S. F. Nabavi, N. Braidy, and S. M. Nabavi (2017) Neuroprotective effects of honokiol: from chemistry to medicine. Biofactors 43: 760–769.
Rauf, A., S. Patel, M. Imran, A. Maalik, M. U. Arshad, F. Saeed, Y. N. Mabkhot, S. S. Al-Showiman, N. Ahmad, and E. Elsharkawy (2018) Honokiol: an anticancer lignan. Biomed. Pharmacother. 107: 555–562.
Sarrica, A., N. Kirika, M. Romeo, M. Salmona, and L. Diomede (2018) Safety and toxicology of magnolol and honokiol. Planta Med. 84: 1151–1164.
Liu, X., Y. Gu, Y. Bian, D. Cai, Y. Li, Y. Zhao, Z. Zhang, M. Xue, and L. Zhang (2021) Honokiol induces paraptosis-like cell death of acute promyelocytic leukemia via mTOR & MAPK signaling pathways activation. Apoptosis 26: 195–208.
Huang, K., Y. Chen, R. Zhang, Y. Wu, Y. Ma, X. Fang, and S. Shen (2018) Honokiol induces apoptosis and autophagy via the ROS/ERK1/2 signaling pathway in human osteosarcoma cells in vitro and in vivo. Cell Death Dis. 9: 157.
Ye, H. and Y. Meng (2021) Honokiol regulates endoplasmic reticulum stress by promoting the activation of the sirtuin 1-mediated protein kinase B pathway and ameliorates high glucose/high fat-induced dysfunction in human umbilical vein endothelial cells. Endocr. J. 68: 981–992.
Liao, G., Z. Zhao, H. Yang, and X. Li (2020) Honokiol ameliorates radiation-induced brain injury via the activation of SIRT3. J. Int. Med. Res. 48: 300060520963993.
Park, C., S. H. Choi, J. W. Jeong, M. H. Han, H. Lee, S. H. Hong, G. Y. Kim, S. K. Moon, W. J. Kim, and Y. H. Choi (2019) Honokiol ameliorates oxidative stress-induced DNA damage and apoptosis of c2c12 myoblasts by ROS generation and mitochondrial pathway. Anim. Cells Syst. (Seoul) 24: 60–68.
Liu, Y., J. Zhou, Y. Luo, J. Li, L. Shang, F. Zhou, and S. Yang (2021) Honokiol alleviates LPS-induced acute lung injury by inhibiting NLRP3 inflammasome-mediated pyroptosis via Nrf2 activation in vitro and in vivo. Chin. Med. 16: 127.
Zhai, T., W. Xu, Y. Liu, K. Qian, Y. Xiong, and Y. Chen (2020) Honokiol alleviates methionine-choline deficient diet-induced hepatic steatosis and oxidative stress in C57BL/6 mice by regulating CFLAR-JNK pathway. Oxid. Med. Cell. Longev. 2020: 2313641.
Vavilala, D. T., V. K. Ponnaluri, D. Kanjilal, and M. Mukherji (2014) Evaluation of anti-HIF and anti-angiogenic properties of honokiol for the treatment of ocular neovascular diseases. PLoS One 9: e113717.
Kim, M. Y., Y. W. Choi, and H. S. Hwang (2021) Regulatory effect on skin differentiation by mevastatin in psoriasis model using TNF-α and IL-17 induced HaCaT cells. Biotechnol. Bioprocess Eng. 26: 348–358.
Noh, Y., J. H. Ahn, J. W. Lee, J. Hong, T. K. Lee, B. Kim, S. S. Kim, and M. H. Won (2020) Brain Factor-7® improves learning and memory deficits and attenuates ischemic brain damage by reduction of ROS generation in stroke in vivo and in vitro. Lab. Anim. Res. 36: 24.
Kim, M. and A. Moon (2021) A curcumin analog CA-5f inhibits urokinase-type plasminogen activator and invasive phenotype of triple-negative breast cancer cells. Toxicol. Res. 38: 19–26.
Volobaev, V. P., E. S. Serdyukova, E. E. Kalyuzhnaya, E. A. Schetnikova, A. D. Korotkova, A. A. Naik, S. N. Bach, A. Y. Prosekov, and A. V. Larionov (2020) Investigation of the genotoxic effects of fluoride on a bone tissue model. Toxicol. Res. 36: 337–342.
Lee, H., D. H. Kim, J. H. Kim, S. K. Park, J. W. Jeong, M. Y. Kim, S. H. Hong, K. S. Song, G. Y. Kim, J. W. Hyun, and Y. H. Choi (2021) Urban aerosol particulate matter promotes necrosis and autophagy via reactive oxygen species-mediated cellular disorders that are accompanied by cell cycle arrest in retinal pigment epithelial cells. Antioxidants (Basel) 10: 149.
Sim, K. H., M.-S. Shu, S. Kim, J.-Y. Kim, B.-Y. Choi, and Y. J. Lee (2021) Cilostazol induces apoptosis and inhibits proliferation of hepatocellular carcinoma cells by activating AMPK. Biotechnol. Bioprocess Eng. 26: 776–785.
Liu, Z., G. Ouyang, W. Lu, and H. Zhang (2021) Long non-coding RNA HOTAIR promotes hepatocellular carcinoma progression by regulating miR-526b-3p/DHX33 axis. Genes Genomics 43: 857–868.
Zhao, B., Z. Wang, J. Han, G. Wei, B. Yi, and Z. Li (2020) Rhizoma Paridis total saponins alleviate H2O2-induced oxidative stress injury by upregulating the Nrf2 pathway. Mol. Med. Rep. 21: 220–228.
Szabo, I., M. Zoratti, and L. Biasutto (2021) Targeting mitochondrial ion channels for cancer therapy. Redox Biol. 42: 101846.
Kim, D. H., J. H. Kim, H. Hwangbo, S. Y. Kim, S. Y. Ji, M. Y. Kim, H. J. Cha, C. Park, S. H. Hong, G. Y. Kim, S. K. Park, J. W. Jeong, M. Y. Kim, Y. H. Choi, and H. Lee (2021) Spermidine attenuates oxidative stress-induced apoptosis via blocking Ca2+ overload in retinal pigment epithelial cells independently of ROS. Int. J. Mol. Sci. 22: 1361.
Liu, H., W. Liu, X. Zhou, C. Long, X. Kuang, J. Hu, Y. Tang, L. Liu, J. He, Z. Huang, Y. Fan, G. Jin, Q. Zhang, and H. Shen (2017) Protective effect of lutein on ARPE-19 cells upon H2O2-induced G2/M arrest. Mol. Med. Rep. 16: 2069–2074.
Dammak, A., F. Huete-Toral, C. Carpena-Torres, A. Martin-Gil, C. Pastrana, and G. Carracedo (2021) From oxidative stress to inflammation in the posterior ocular diseases: diagnosis and treatment. Pharmaceutics 13: 1376.
Chan, T. C., J. L. Wilkinson Berka, D. Deliyanti, D. Hunter, A. Fung, G. Liew, and A. White (2020) The role of reactive oxygen species in the pathogenesis and treatment of retinal diseases. Exp. Eye Res. 201: 108255.
Tang, P., J. M. Gu, Z. A. Xie, Y. Gu, Z. W. Jie, K. M. Huang, J. Y. Wang, S. W. Fan, X. S. Jiang, and Z. J. Hu (2018) Honokiol alleviates the degeneration of intervertebral disc via suppressing the activation of TXNIP-NLRP3 inflammasome signal pathway. Free Radic. Biol. Med. 120: 368–379.
Mahendra, C. K. L. T. H. Tan, P. Pusparajah, T. T. Htar, L. H. Chuah, V. S. Lee, L. E. Low, S. Y. Tang, K. G. Chan, and B. H. Goh (2020) Detrimental effects of UVB on retinal pigment epithelial cells and its role in age-related macular degeneration. Oxid. Med. Cell. Longev. 2020: 1904178.
Park, C., H. Lee, S. H. Hong, J. H. Kim, S. K. Park, J. W. Jeong, G. Y. Kim, J. W. Hyun, S. J. Yun, B. W. Kim, W. J. Kim, and Y. H. Choi (2019) Protective effect of diphlorethohydroxycarmalol against oxidative stress-induced DNA damage and apoptosis in retinal pigment epithelial cells. Cutan. Ocul. Toxicol. 38: 298–308.
Ulasov, A. V., A. A. Rosenkranz, G. P. Georgiev, and A. S. Sobolev (2022) Nrf2/Keap1/ARE signaling: towards specific regulation. Life Sci. 291: 120111.
Cai, Z. Y., M. D. Fu, K. Liu, and X. C. Duan (2021) Therapeutic effect of Keap1-Nrf2-ARE pathway-related drugs on age-related eye diseases through anti-oxidative stress. Int. J. Ophthalmol. 14: 1260–1273.
Tavakkoli, A., M. Iranshahi, S. H. Hasheminezhad, A. W. Hayes, and G. Karimi (2019) The neuroprotective activities of natural products through the Nrf2 upregulation. Phytother. Res. 33: 2256–2273.
Zhang, Y., Z. Zhao, X. Zhao, H. Xie, C. Zhang, X. Sun, and J. Zhang (2022) HMGB2 causes photoreceptor death via down-regulating Nrf2/HO-1 and up-regulating NF-κB/NLRP3 signaling pathways in light-induced retinal degeneration model. Free Radic. Biol. Med. 181: 14–28.
Laspas, P., M. B. Zhutdieva, C. Brochhausen, A. Musayeva, J. K. Zadeh, N. Pfeiffer, N. Xia, H. Li, J. Wess, and A. Gericke (2019) The M1 muscarinic acetylcholine receptor subtype is important for retinal neuron survival in aging mice. Sci. Rep. 9: 5222.
Li, L., G. Du, D. Wang, J. Zhou, G. Jiang, and H. Jiang (2017) Overexpression of heme oxygenase-1 in mesenchymal stem cells augments their protection on retinal cells in vitro and attenuates retinal ischemia/reperfusion injury in vivo against oxidative stress. Stem Cells Int. 2017: 4985323.
Dadsena, S., L. E. King, and A. J. García-Sáez (2021) Apoptosis regulation at the mitochondria membrane level. Biochim. Biophys. Acta Biomembr. 1863: 183716.
Senichkin, V. V., N. V. Pervushin, A. P. Zuev, B. Zhivotovsky, and G. S. Kopeina (2020) Targeting Bcl-2 family proteins: what, where, when? Biochemistry (Mosc.) 85: 1210–1226.
Niture, S. K. and A. K. Jaiswal (2012) Nrf2 protein up-regulates antiapoptotic protein Bcl-2 and prevents cellular apoptosis. J. Biol. Chem. 287: 9873–9886.
Rowan, S., S. Jiang, M. L. Chang, J. Volkin, C. Cassalman, K. M. Smith, M. D. Streeter, D. A. Spiegel, C. Moreira-Neto, N. Rabbani, P. J. Thornalley, D. E. Smith, N. K. Waheed, and A. Taylor (2020) A low glycemic diet protects disease-prone Nrf2-deficient mice against age-related macular degeneration. Free Radic. Biol. Med. 150: 75–86.
Zhao, Z., P. Xu, Z. Jie, Y. Zuo, B. Yu, L. Soong, J. Sun, Y. Chen, and J. Cai (2014) γδ T cells as a major source of IL-17 production during age-dependent RPE degeneration. Invest. Ophthalmol. Vis. Sci. 55: 6580–6589.
Zhao, Z., Y. Chen, J. Wang, P. Sternberg, M. L. Freeman, H. E. Grossniklaus, and J. Cai (2011) Age-related retinopathy in NRF2-deficient mice. PLoS One 6: e19456.
Hyttinen, J. M. T., R. Kannan, S. Felszeghy, M. Niittykoski, A. Salminen, and K. Kaarniranta (2019) The regulation of NFE2L2 (NRF2) signalling and epithelial-to-mesenchymal transition in age-related macular degeneration pathology. Int. J. Mol. Sci. 20: 5800.
Bellezza, I. (2018) Oxidative stress in age-related macular degeneration: Nrf2 as therapeutic target. Front. Pharmacol. 9: 1280.
Datta, S., M. Cano, K. Ebrahimi, L. Wang, and J. T. Handa (2017) The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 60: 201–218.
Lambros, M. L. and S. M. Plafker (2016) Oxidative stress and the Nrf2 anti-oxidant transcription factor in age-related macular degeneration. Adv. Exp. Med. Biol. 854: 67–72.
Zhang, J., H. Zhou, J. Chen, X. Lv, and H. Liu (2022) Aloperine protects human retinal pigment epithelial cells against hydrogen peroxide-induced oxidative stress and apoptosis through activation of Nrf2/HO-1 pathway. J. Recept. Signal Transduct. Res. 42: 88–94.
Chen, W., Y. Ye, Z. Wu, J. Lin, Y. Wang, Q. Ding, X. Yang, W. Yang, B. Lin, and B. Lin (2021) Temporary upregulation of Nrf2 by naringenin alleviates oxidative damage in the retina and ARPE-19 cells. Oxid. Med. Cell. Longev. 2021: 4053276.
Clementi, M. E., B. Sampaolese, F. Sciandra, and G. Tringali (2020) Punicalagin protects human retinal pigment epithelium cells from ultraviolet radiation-induced oxidative damage by activating Nrf2/HO-1 signaling pathway and reducing apoptosis. Antioxidants (Basel) 9: 473.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) Grant funded by the Korea government (NRF-2020R1A2C1099910 and NRF-2021R1A2C200954911).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Neither ethical approval nor informed consent was required for this study.
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hong, S.H., Park, C., Hwangbo, H. et al. Activation of Heme Oxygenase-1 is Involved in the Preventive Effect of Honokiol against Oxidative Damage in Human Retinal Pigment Epithelial Cells. Biotechnol Bioproc E 27, 975–986 (2022). https://doi.org/10.1007/s12257-022-0174-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-022-0174-x