Abstract
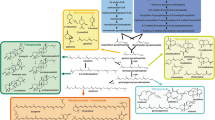
Terpenoids are a large variety of natural products with remarkable diverse biological functions, and have a wide range of applications in flavors, pharmaceuticals, biofuels, pigments, and so on. However, limited production of terpenoids from natural resources constrains their use of bulk commodity products. In vivo synthetic biosystem, harnessing living organisms to produce terpenoids, has been broadly used and in-depth reviewed for terpenoids production, which has inherent weaknesses, such as slow reaction rate, low product yield, toxic intermediates, and high separation cost. In vitro synthetic biosystem, harboring numerous enzymes and/or coenzymes assembled into an in vitro enzymatic bioreactions, can obviate part of problems associated with in vivo style. In this review, the general design of in vitro synthetic biosystem is presented with seven supporting examples: mevalonate, isoprene, limonene, pinene, farnesene, amorpha-4,11-diene and taxadiene. The efforts for the large-scale implementation of in vitro synthetic biosystem have been addressed to enzymes engineering, computational modeling and cofactors recycle. The review also discusses the challenges and solutions for the large-scale implementation of in vitro synthetic biosystem around enzymes stability and cofactors recycle. This review may suggest in vitro synthetic biosystems become a realistic option for the production of diverse valuable terpenoids, even expand to other commodity chemicals.
Similar content being viewed by others
Change history
24 December 2022
An Erratum to this paper has been published: https://doi.org/10.1007/s12257-022-1111-8
Abbreviations
- ACAT :
-
acetyl-CoA acetyltransferase
- HMGS :
-
hydroxymethylglutaryl-CoA synthase
- HMGR :
-
hydroxymethylglutaryl-CoA reductase
- ackA :
-
acetate kinase
- pta :
-
phosphate acetyltransferase
- G6PDH :
-
glucose-6-phosphate dehydrogenase
- GsPDH :
-
pyruvate dehydrogenase from Geobacillus stearothermophilus, which is specific for NAD+ to produce acetyl-CoA
- LlNoxE :
-
NADH oxidase from Lactobacillus lactis
- GsPyk :
-
pyruvate kinase from G. stearothermophilus
- CoPASI:
-
a biochemical simulator
- Hex:
-
hexokinase
- Pdh :
-
pyruvate dehydrogenase
- Pi :
-
inorganic phosphate
- ck :
-
choline kinase
- ipk :
-
isopentenyl kinase
- idi :
-
isopentenyl pyrophosphate isomerase
- ispA :
-
farnesyl pyrophosphate synthase
- ggpps :
-
geranylgeranyl pyrophosphate synthase
- tds :
-
taxadiene synthase.
- GsPDH/LlNoxE system:
-
the system recycles free CoA and produce acetyl-CoA continuously.
- Plackett-Burman experimental design:
-
this method is a technique for the optimization of reaction components.
- Taguchi method:
-
this method is an engineering method with low cost and high benefit. For the purpose of experimental design, this method describes a large number of experimental situations to reduce experimental errors and enhance the efficiency and reproducibility of laboratory experiments.
- ackA-pta pathway:
-
this pathway plays part in acetate metabolism.
References
Wang, C., B. Zada, G. Wei, and S. W. Kim (2017) Metabolic engineering and synthetic biology approaches driving isoprenoid production in Escherichia coli. Bioresour. Technol. 241: 430–438.
Zyad, A., M. Tilaoui, A. Jaafari, M. A. Oukerrou, and H. A. Mouse (2018) More insights into the pharmacological effects of artemisinin. Phytother. Res. 32: 216–229.
Liu, T. and C. Khosla (2010) Chemistry. A balancing act for Taxol precursor pathways in E. coli. Science. 330: 44–45.
Wang, C., M. Liwei, J. B. Park, S. H. Jeong, G. Wei, Y. Wang, and S. W. Kim (2018) Microbial platform for terpenoid production: Escherichia coli and yeast. Front. Microbiol. 9: 2460.
Liao, P., A. Hemmerlin, T. J. Bach, and M. L. Chye (2016) The potential of the mevalonate pathway for enhanced isoprenoid production. Biotechnol. Adv. 34: 697–713.
Yang, L., C. Wang, J. Zhou, and S. W. Kim (2016) Combinatorial engineering of hybrid mevalonate pathways in Escherichia coli for protoilludene production. Microb. Cell Fact. 15: 14.
Martin, V. J., D. J. Pitera, S. T. Withers, J. D. Newman, and J. D. Keasling (2003) Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat. Biotechnol. 21: 796–802.
Lv, X., F. Wang, P. Zhou, L. Ye, W. Xie, H. Xu, and H. Yu (2016) Dual regulation of cytoplasmic and mitochondrial acetyl-CoA utilization for improved isoprene production in Saccharomyces cerevisiae. Nat. Commun. 7: 12851.
Meadows, A. L., K. M. Hawkins, Y. Tsegaye, E. Antipov, Y. Kim, L. Raetz, R. H. Dahl, A. Tai, T. Mahatdejkul-Meadows, L. Xu, L. Zhao, M. S. Dasika, A. Murarka, J. Lenihan, D. Eng, J. S. Leng, C. L. Liu, J. W. Wenger, H. Jiang, L. Chao, P. Westfall, J. Lai, S. Ganesan, P. Jackson, R. Mans, D. Platt, C. D. Reeves, P. R. Saija, G. Wichmann, V. F. Holmes, K. Benjamin, P. W. Hill, T. S. Gardner, and A. E. Tsong (2016) Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature. 537: 694–697.
Xia, P. F., H. Ling, J. L. Foo, and M. W. Chang (2019) Synthetic genetic circuits for programmable biological functionalities. Biotechnol. Adv. 37: 107393.
Biggs, B. W., C. G. Lim, K. Sagliani, S. Shankar, G. Stephanopoulos, M. De Mey, and P. K. Ajikumar (2016) Overcoming heterologous protein interdependency to optimize P450-mediated Taxol precursor synthesis in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 113: 3209–3214.
Nielsen, J. and J. D. Keasling (2016) Engineering cellular metabolism. Cell. 164: 1185–1197.
Zhang, C. and K. Hong (2020) Production of terpenoids by synthetic biology approaches. Front. Bioeng. Biotechnol. 8: 347.
Sarria, S., B. Wong, H. García Martín, J. D. Keasling, and P. Peralta-Yahya (2014) Microbial synthesis of pinene. ACS Synth. Biol. 3: 466–475.
Zhang, Y. H. (2015) Production of biofuels and biochemicals by in vitro synthetic biosystems: opportunities and challenges. Biotechnol. Adv. 33: 1467–1483.
Opgenorth, P. H., T. P. Korman, and J. U. Bowie (2014) A synthetic biochemistry molecular purge valve module that maintains redox balance. Nat. Commun. 5: 4113.
Zhang, Y. H. (2010) Production of biocommodities and bioelectricity by cell-free synthetic enzymatic pathway biotransformations: challenges and opportunities. Biotechnol. Bioeng. 105: 663–677.
Karim, A. S., Q. M. Dudley, A. Juminaga, Y. Yuan, S. A. Crowe, J. T. Heggestad, S. Garg, T. Abdalla, W. S. Grubbe, B. J. Rasor, D. N. Coar, M. Torculas, M. Krein, F. E. Liew, A. Quattlebaum, R. O. Jensen, J. A. Stuart, S. D. Simpson, M. Köpke, and M. C. Jewett (2020) In vitro prototyping and rapid optimization of biosynthetic enzymes for cell design. Nat. Chem. Biol. 16: 912–919.
Kizer, L., D. J. Pitera, B. F. Pfleger, and J. D. Keasling (2008) Application of functional genomics to pathway optimization for increased isoprenoid production. Appl. Environ. Microbiol. 74: 3229–3241.
You, C., T. Shi, Y. Li, P. Han, X. Zhou, and Y. P. Zhang (2017) An in vitro synthetic biology platform for the industrial biomanufacturing of myo-inositol from starch. Biotechnol. Bioeng. 114: 1855–1864.
Dudley, Q. M., A. S. Karim, and M. C. Jewett (2015) Cell-free metabolic engineering: biomanufacturing beyond the cell. Biotechnol. J. 10: 69–82.
Dudley, Q. M., K. C. Anderson, and M. C. Jewett (2016) Cell-free mixing of Escherichia coli crude extracts to prototype and rationally engineer high-titer mevalonate synthesis. ACS Synth. Biol. 5: 1578–1588.
Korman, T. P., B. Sahachartsiri, D. Li, J. M. Vinokur, D. Eisenberg, and J. U. Bowie (2014) A synthetic biochemistry system for the in vitro production of isoprene from glycolysis intermediates. Protein Sci. 23: 576–585.
Zhu, F., X. Zhong, M. Hu, L. Lu, Z. Deng, and T. Liu (2014) In vitro reconstitution of mevalonate pathway and targeted engineering of farnesene overproduction in Escherichia coli. Biotechnol. Bioeng. 111: 1396–1405.
Chen, X., C. Zhang, R. Zou, K. Zhou, G. Stephanopoulos, and H. P. Too (2013) Statistical experimental design guided optimization of a one-pot biphasic multienzyme total synthesis of amorpha-4,11-diene. PLoS One. 8: e79650.
Ward, V. C. A., A. O. Chatzivasileiou, and G. Stephanopoulos (2019) Cell free biosynthesis of isoprenoids from isopentenol. Biotechnol. Bioeng. 116: 3269–3281.
You, C. and Y. H. Zhang (2013) Cell-free biosystems for biomanufacturing. Adv. Biochem. Eng. Biotechnol. 131: 89–119.
Opgenorth, P. H., T. P. Korman, and J. U. Bowie (2016) A synthetic biochemistry module for production of bio-based chemicals from glucose. Nat. Chem. Biol. 12: 393–395.
Ye, X., K. Honda, T. Sakai, K. Okano, T. Omasa, R. Hirota, A. Kuroda, and H. Ohtake (2012) Synthetic metabolic engineering-a novel, simple technology for designing a chimeric metabolic pathway. Microb. Cell Fact. 11: 120.
Frank, A. and M. Groll (2017) The methylerythritol phosphate pathway to isoprenoids. Chem. Rev. 117: 5675–5703.
Satowa, D., R. Fujiwara, S. Uchio, M. Nakano, C. Otomo, Y. Hirata, T. Matsumoto, S. Noda, T. Tanaka, and A. Kondo (2020) Metabolic engineering of E. coli for improving mevalonate production to promote NADPH regeneration and enhance acetyl-CoA supply. Biotechnol. Bioeng. 117: 2153–2164.
Wegner, S. A., J. M. Chen, S. S. Ip, Y. Zhang, D. Dugar, and J. L. Avalos (2021) Engineering acetyl-CoA supply and ERG9 repression to enhance mevalonate production in Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 48: kuab050.
Lindberg, P., S. Park, and A. Melis (2010) Engineering a platform for photosynthetic isoprene production in cyanobacteria, using Synechocystis as the model organism. Metab. Eng. 12: 70–79.
Chenault, H. K. and G. M. Whitesides (1987) Regeneration of nicotinamide cofactors for use in organic synthesis. Appl. Biochem. Biotechnol. 14: 147–197.
Lau, M. W., C. Gunawan, V. Balan, and B. E. Dale (2010) Comparing the fermentation performance of Escherichia coli KO11, Saccharomyces cerevisiae 424A(LNH-ST) and Zymomonas mobilis AX101 for cellulosic ethanol production. Biotechnol. Biofuels. 3: 11.
Mukhtar, Y. M., M. Adu-Frimpong, X. Xu, and J. Yu (2018) Biochemical significance of limonene and its metabolites: future prospects for designing and developing highly potent anticancer drugs. Biosci. Rep. 38: BSR20181253.
Alonso-Gutierrez, J., R. Chan, T. S. Batth, P. D. Adams, J. D. Keasling, C. J. Petzold, and T. S. Lee (2013) Metabolic engineering of Escherichia coli for limonene and perillyl alcohol production. Metab. Eng. 19: 33–41.
Jongedijk, E., K. Cankar, M. Buchhaupt, J. Schrader, H. Bouwmeester, and J. Beekwilder (2016) Biotechnological production of limonene in microorganisms. Appl. Microbiol. Biotechnol. 100: 2927–2938.
Cao, X., Y. B. Lv, J. Chen, T. Imanaka, L. J. Wei, and Q. Hua (2016) Metabolic engineering of oleaginous yeast Yarrowia lipolytica for limonene overproduction. Biotechnol. Biofuels. 9: 214.
Cheng, S., X. Liu, G. Jiang, J. Wu, J. L. Zhang, D. Lei, Y. J. Yuan, J. Qiao, and G. R. Zhao (2019) Orthogonal engineering of biosynthetic pathway for efficient production of limonene in Saccharomyces cerevisiae. ACS Synth. Biol. 8: 968–975.
Davies, F. K., V. H. Work, A. S. Beliaev, and M. C. Posewitz (2014) Engineering limonene and bisabolene production in wild type and a glycogen-deficient mutant of Synechococcus sp. PCC 7002. Front. Bioeng. Biotechnol. 2: 21.
Chubukov, V., F. Mingardon, W. Schackwitz, E. E. Baidoo, J. Alonso-Gutierrez, Q. Hu, T. S. Lee, J. D. Keasling, and A. Mukhopadhyay (2015) Acute limonene toxicity in Escherichia coli is caused by limonene hydroperoxide and alleviated by a point mutation in alkyl hydroperoxidase AhpC. Appl. Environ. Microbiol. 81: 4690–4696.
Tomko, T. A. and M. J. Dunlop (2015) Engineering improved bio-jet fuel tolerance in Escherichia coli using a transgenic library from the hydrocarbon-degrader Marinobacter aquaeolei. Biotechnol. Biofuels. 8: 165.
Korman, T. P., P. H. Opgenorth, and J. U. Bowie (2017) A synthetic biochemistry platform for cell free production of monoterpenes from glucose. Nat. Commun. 8: 15526.
Hoops, S., S. Sahle, R. Gauges, C. Lee, J. Pahle, N. Simus, M. Singhal, L. Xu, P. Mendes, and U. Kummer (2006) COPASI—a Complex pathway simulator. Bioinformatics. 22: 3067–3074.
Willrodt, C., C. David, S. Cornelissen, B. Bühler, M. K. Julsing, and A. Schmid (2014) Engineering the productivity of recombinant Escherichia coli for limonene formation from glycerol in minimal media. Biotechnol. J. 9: 1000–1012.
Rolf, J., M. K. Julsing, K. Rosenthal, and S. Lütz (2020) A gram-scale limonene production process with engineered Escherichia coli. Molecules. 25: 1881.
Dudley, Q. M., A. S. Karim, C. J. Nash, and M. C. Jewett (2020) In vitro prototyping of limonene biosynthesis using cell-free protein synthesis. Metab. Eng. 61: 251–260.
Gomes-Carneiro, M. R., M. E. Viana, I. Felzenszwalb, and F. J. Paumgartten (2005) Evaluation of beta-myrcene, alpha-terpinene and (+)- and (−)-alpha-pinene in the Salmonella/microsome assay. Food Chem. Toxicol. 43: 247–252.
Kirby, J. and J. D. Keasling (2009) Biosynthesis of plant isoprenoids: perspectives for microbial engineering. Annu. Rev. Plant Biol. 60: 335–355.
Yang, J., Q. Nie, M. Ren, H. Feng, X. Jiang, Y. Zheng, M. Liu, H. Zhang, and M. Xian (2013) Metabolic engineering of Escherichia coli for the biosynthesis of alpha-pinene. Biotechnol. Biofuels. 6: 60.
Tashiro, M., H. Kiyota, S. Kawai-Noma, K. Saito, M. Ikeuchi, Y. Iijima, and D. Umeno (2016) Bacterial production of pinene by a laboratory-evolved pinene-synthase. ACS Synth. Biol. 5: 1011–1020.
Niu, F. X., Y. B. Huang, Y. P. Shen, L. N. Ji, and J. Z. Liu (2020) Enhanced production of pinene by using a cell-free system with modular cocatalysis. J. Agric. Food Chem. 68: 2139–2145.
Niu, F. X., X. He, Y. Q. Wu, and J. Z. Liu (2018) Enhancing production of pinene in Escherichia coli by using a combination of tolerance, evolution, and modular co-culture engineering. Front. Microbiol. 9: 1623.
Yang, T., G. Stoopen, N. Yalpani, J. Vervoort, R. de Vos, A. Voster, F. W. Verstappen, H. J. Bouwmeester, and M. A. Jongsma (2011) Metabolic engineering of geranic acid in maize to achieve fungal resistance is compromised by novel glycosylation patterns. Metab. Eng. 13: 414–425.
Wang, C., S. H. Yoon, H. J. Jang, Y. R. Chung, J. Y. Kim, E. S. Choi, and S. W. Kim (2011) Metabolic engineering of Escherichia coli for α-farnesene production. Metab. Eng. 13: 648–655.
Tsuruta, H., C. J. Paddon, D. Eng, J. R. Lenihan, T. Horning, L. C. Anthony, R. Regentin, J. D. Keasling, N. S. Renninger, and J. D. Newman (2009) High-level production of amorpha-4,11-diene, a precursor of the antimalarial agent artemisinin, in Escherichia coli. PLoS One. 4: e4489.
Ro, D. K., E. M. Paradise, M. Ouellet, K. J. Fisher, K. L. Newman, J. M. Ndungu, K. A. Ho, R. A. Eachus, T. S. Ham, J. Kirby, M. C. Chang, S. T. Withers, Y. Shiba, R. Sarpong, and J. D. Keasling (2006) Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature. 440: 940–943.
Chen, X., C. Zhang, R. Zou, G. Stephanopoulos, and H. P. Too (2017) In vitro metabolic engineering of amorpha-4,11-diene biosynthesis at enhanced rate and specific yield of production. ACS Synth. Biol. 6: 1691–1700.
Ajikumar, P. K., W. H. Xiao, K. E. Tyo, Y. Wang, F. Simeon, E. Leonard, O. Mucha, T. H. Phon, B. Pfeifer, and G. Stephanopoulos (2010) Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science. 330: 70–74.
Kay, J. E. and M. C. Jewett (2020) A cell-free system for production of 2,3-butanediol is robust to growth-toxic compounds. Metab. Eng. Commun. 10: e00114.
Bujara, M., M. Schümperli, S. Billerbeck, M. Heinemann, and S. Panke (2010) Exploiting cell-free systems: implementation and debugging of a system of biotransformations. Biotechnol. Bioeng. 106: 376–389.
Kay, J. E. and M. C. Jewett (2015) Lysate of engineered Escherichia coli supports high-level conversion of glucose to 2,3-butanediol. Metab. Eng. 32: 133–142.
Zhang, Y. H. (2011) Substrate channeling and enzyme complexes for biotechnological applications. Biotechnol. Adv. 29: 715–725.
Ge, J., J. Lei, and R. N. Zare (2012) Protein-inorganic hybrid nanoflowers. Nat. Nanotechnol. 7: 428–432.
Myung, S. and Y. H. Zhang (2013) Non-complexed four cascade enzyme mixture: simple purification and synergetic co-stabilization. PLoS One. 8: e61500.
Echave, J. (2019) Beyond stability constraints: a biophysical model of enzyme evolution with selection on stability and activity. Mol. Biol. Evol. 36: 613–620.
Kim, J. E. and Y. H. Zhang (2016) Biosynthesis of D-xylulose 5-phosphate from D-xylose and polyphosphate through a minimized two-enzyme cascade. Biotechnol. Bioeng. 113: 275–282.
Liu, Y., J. Wang, C. Xu, Y. Chen, J. Yang, D. Liu, H. Niu, Y. Jiang, S. Yang, and H. Ying (2017) Efficient multi-enzyme-catalyzed CDP-choline production driven by an ATP donor module. Appl. Microbiol. Biotechnol. 101: 1409–1417.
Kim, E. M., H. M. Woo, T. Tian, S. Yilmaz, P. Javidpour, J. D. Keasling, and T. S. Lee (2017) Autonomous control of metabolic state by a quorum sensing (QS)-mediated regulator for bisabolene production in engineered E. coli. Metab. Eng. 44: 325–336.
Acknowledgements
This work is supported by the Research Project Supported by Shanxi Scholarship Council of China (No. 2020-132), the Jinzhong Key Research and Development Program for Science and Technology (No. Y191021), the Science and Technology Innovation Project of Universities in Shanxi province (No. 2019L0721), and the Basic Research Project of Shanxi University of Traditional Chinese Medicine (No. 2020PY-JC(Y)-05).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare no conflict of interest.
Neither ethical approval nor informed consent was required for this study.
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liyang, Y., Qiang, G., Jifang, L. et al. Opportunities and Challenges of in vitro Synthetic Biosystem for Terpenoids Production. Biotechnol Bioproc E 27, 697–705 (2022). https://doi.org/10.1007/s12257-022-0100-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-022-0100-2