Abstract
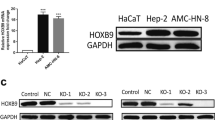
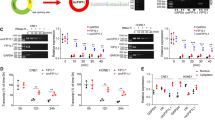
Cholesteatoma is a pathologically benign but clinically destructive middle ear disease characterized by hyperproliferative keratinocytes. B-cell-specific Moloney murine leukemia virus insertion site 1 (BMI1) has been reported to be upregulated in cholesteatoma tissues. This study aimed to explore the biological role and underlying mechanisms of BMI1 in the progression of cholesteatoma. The expression levels of microRNA (miR)-1297, miR-26a- 5p, and BMI1 in cholesteatoma tissues and cells were examined by quantitative real-time polymerase chain reaction (qRT-PCR) or western blot. Functional experiments were performed by CCK-8 assay for cell proliferation viability, 5-ethynyl-2'deoxyuridine (EdU) incorporation assay for DNA biosynthesis, colony formation assay for cloning forming ability analysis, transwell assay and wound healing assay for cell metastasis, flow cytometry for cell cycle distribution and cell apoptosis. The protein expression of apoptosis-associated proteins was investigated by western blot. Dual-luciferase reporter assay was conducted to verify the interaction between miR-1297 or miR-26a-5p and BMI1. BMI1 was highly expressed in cholesteatoma tumor tissues. Functional analyses showed that BMI1 knockdown could inhibit the proliferation, colony formation, migration, invasion, cell cycle progression and promoted the apoptosis of keratinocytes. Mechanically, BMI1 was a target of miR-1297 and miR-26a-5p. Moreover, the rescue experiments presented that BMI1 addition could abolish the suppressive effects of miR-1297 or miR-26a-5p overexpression on cell malignant behaviors in keratinocytes. BMI1 could exert an oncogenic role in the malignant development of cholesteatoma through serving as the targets of miR-1297 and miR-26a-5p, which might provide novel strategies for cholesteatoma treatment.
Similar content being viewed by others
References
Sculerati, N. and C. D. Bluestone (1989) Pathogenesis of cholesteatoma. Otolaryngol. Clin. North. Am. 22: 859–868.
Maniu, A., O. Harabagiu, M. Perde Schrepler, A. Cătană, B. Fănuţă, and C. A. Mogoantă (2014) Molecular biology of cholesteatoma. Rom. J. Morphol. Embryol. 55: 7–13.
Lau, K., M. Stavrakas, M. Yardley, and J. Ray (2021) Lasers in cholesteatoma surgery: A systematic review. Ear Nose Throat J. 100: 94S–99S.
Palva, T. (1990) The pathogenesis and treatment of cholesteatoma. Acta Otolaryngol. 109: 323–330.
Kim, H. J., S. P. Tinling, and R. A. Chole (2002) Increased proliferation and migration of epithelium in advancing experimental cholesteatomas. Otol. Neurotol. 23: 840–844.
Kim, H. J., S. P. Tinling, and R. A. Chole (2002) Expression patterns of cytokeratins in cholesteatomas: evidence of increased migration and proliferation. J. Korean Med. Sci. 17: 381–388.
Mo, Y. Y. (2012) MicroRNA regulatory networks and human disease. Cell. Mol. Life Sci. 69: 3529–3531.
Lu, T. X. and M. E. Rothenberg (2018) MicroRNA. J. Allergy Clin. Immunol. 141: 1202–1207.
Di Leva, G., M. Garofalo, and C. M. Croce (2014) MicroRNAs in cancer. Annu. Rev. Pathol. 9: 287–314.
Kontomanolis, E. N., A. Koukouli, G. Liberis, H. Stanulov, A. Achouhan, and A. Pagkalos (2016) MiRNAs: regulators of human disease. Eur. J. Gynaecol. Oncol. 37: 759–765.
Mahmoudian-Sani, M. R., A. Mehri-Ghahfarrokhi, F. Ahmadinejad, M. Hashemzadeh-Chaleshtori, M. Saidijam, and M. S. Jami (2017) MicroRNAs: effective elements in ear-related diseases and hearing loss. Eur. Arch. Otorhinolaryngol. 274: 2373–2380.
Xie, S., X. Liu, Z. Pan, X. Chen, A. Peng, T. Yin, J. Ren, and W. Liu (2018) Microarray analysis of differentially-expressed microRNAs in acquired middle ear cholesteatoma. Int. J. Med. Sci. 15: 1547–1554.
Zhang, W., X. Chen, and Z. Qin (2015) MicroRNA let-7a suppresses the growth and invasion of cholesteatoma keratinocytes. Mol. Med. Rep. 11: 2097–2103.
Li, N. and Z. B. Qin (2014) Inflammation-induced miR-802 promotes cell proliferation in cholesteatoma. Biotechnol. Lett. 36: 1753–1759.
Yang, D., H. Q. Liu, Z. Yang, D. Fan, and Q. Z. Tang (2021) BMI1 in the heart: Novel functions beyond tumorigenesis. EBioMedicine. 63: 103193.
Banerjee Mustafi, S., P. K. Chakraborty, S. K. D. Dwivedi, K. Ding, K. M. Moxley, P. Mukherjee, and R. Bhattacharya} (2017) BMI1, a new target of CK2alpha. Mol. Cancer. 16: 56.
Zang, J., L. Hui, N. Yang, B. Yang, and X. Jiang (2018) Downregulation of MiR-203a disinhibits Bmi1 and promotes growth and proliferation of keratinocytes in cholesteatoma. Int. J. Med. Sci. 15: 447–455.
Bhutta, M. F., I. G. Williamson, and H. H. Sudhoff (2011) Cholesteatoma. BMJ. 342: d1088.
Ren, H., P. Du, Z. Ge, Y. Jin, D. Ding, X. Liu, and Q. Zou (2016) TWIST1 and BMI1 in cancer metastasis and chemoresistance. J. Cancer. 7: 1074–1080.
Wu, C. Y., J. J. Hung, and K. J. Wu (2012) Linkage between Twist1 and Bmi1: molecular mechanism of cancer metastasis/stemness and clinical implications. Clin. Exp. Pharmacol. Physiol. 39: 668–373.
Chen, D., L. Cheng, H. Cao, and W. Liu (2021) Role of microRNA-381 in bladder cancer growth and metastasis with the involvement of BMI1 and the Rho/ROCK axis. BMC Urol. 21: 5.
Guo, J., N. Deng, Y. Xu, L. Li, D. Kuang, M. Li, X. Li, Z. Xu, M. Xiang, and C. Xu (2021) Bmi1 drives the formation and development of intrahepatic cholangiocarcinoma independent of Ink4A/Arf repression. Pharmacol. Res. 164: 105365.
Lee, K., G. Adhikary, S. Balasubramanian, R. Gopalakrishnan, T. McCormick, G. P. Dimri, R. L. Eckert, and E. A. Rorke (2008) Expression of Bmi-1 in epidermis enhances cell survival by altering cell cycle regulatory protein expression and inhibiting apoptosis. J. Invest. Dermatol. 128: 9–17.
Balasubramanian, S., G. Adhikary, and R. L. Eckert (2010) The Bmi-1 polycomb protein antagonizes the (-)-epigallocatechin-3- gallate-dependent suppression of skin cancer cell survival. Carcinogenesis. 31: 496–503.
Lee, Y. S. and A. Dutta (2009) MicroRNAs in cancer. Annu. Rev. Pathol. 4: 199–227.
Kabekkodu, S. P., V. Shukla, V. K. Varghese, J. D’ Souza, S. Chakrabarty, and K. Satyamoorthy (2018) Clustered miRNAs and their role in biological functions and diseases. Biol. Rev. Camb. Philos. Soc. 93: 1955–1986.
Chen, X., X. Li, and Z. Qin (2016) MicroRNA-21 promotes the proliferation and invasion of cholesteatoma keratinocytes. Acta Otolaryngol. 136: 1261–1266.
Wang, Y., X. Liu, L. Wang, Z. Zhang, Z. Li, and M. Li (2021) Circ_PGPEP1 serves as a sponge of miR-1297 to promote gastric cancer progression via regulating E2F3. Dig. Dis. Sci. 66: 4302–4313.
Pan, X., H. Li, J. Tan, X. Weng, L. Zhou, Y. Weng, and X. Cao (2020) miR-1297 suppresses osteosarcoma proliferation and aerobic glycolysis by regulating PFKFB2. Onco. Targets Ther. 13: 11265–11275.
Wang, Y., J. Xue, H. Kuang, X. Zhou, L. Liao, and F. Yin (2017) microRNA-1297 inhibits the growth and metastasis of colorectal cancer by suppressing cyclin D2 expression. DNA Cell Biol. 36: 991–999.
Wang, J., J. Ni, D. Song, M. Ding, J. Huang, W. Li, and G. He (2020) The regulatory effect of has-circ-0001146/miR-26a-5p/ MNAT1 network on the proliferation and invasion of osteosarcoma. Biosci Rep. 40: BSR20201232.
Zhang, X. M., J. Wang, Z. L. Liu, H. Liu, Y. F. Cheng, and T. Wang (2020) LINC00657/miR-26a-5p/CKS2 ceRNA network promotes the growth of esophageal cancer cells via the MDM2/ p53/Bcl2/Bax pathway. Biosci Rep. 40: BSR20200525.
Lv, P., X. Qiu, Y. Gu, X. Yang, X. Xu, and Y. Yang (2019) Long non-coding RNA SNHG6 enhances cell proliferation, migration and invasion by regulating miR-26a-5p/MAPK6 in breast cancer. Biomed Pharmacother. 110: 294–301.
Zhou, J., Y. Liu, Z. Ren, Y. Zhang, and M. Zhang (2017) Transarterial chemoembolization with gelatin sponge microparticles for barcelona clinic liver cancer Stage C and large hepatocellular carcinoma: Initial clinical experience. J. Cancer Res. Ther. 13: 767–772.
Chole, R. A., R. M. Hughes, and B. T. Faddis (2001) Keratin particle-induced osteolysis: a mouse model of inflammatory bone remodeling related to cholesteatoma. J. Assoc. Res. Otolaryngol. 2: 65–71.
Acknowledgement
None
Funding
There is no funding to report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All participates had signed informed consents prior to surgery and this research was proceeded with the permission of the Ethics Committee of The First Affiliated Hospital of Zhengzhou University.
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Disclosure of Interest
The authors declare that they have no conflicts of interest.
Data Availability Statements
Data analyzed for this study will be available on a reasonable request.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Zhu, X., Ye, F., Hao, S. et al. MiR-1297 and MiR-26a-5p Inhibit Cell Progression of Keratinocytes in Cholesteatoma Depending on the Regulation of BMI1. Biotechnol Bioproc E 27, 79–88 (2022). https://doi.org/10.1007/s12257-021-0178-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-021-0178-y