Abstract
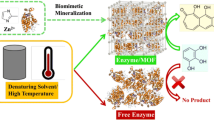
As a series of metal organic framework (MOFs), materials of institute lavoisier frameworks (MILs) are expected to be excellent supports for enzyme immobilization due to their good water-stability and acid tolerance. However, enzyme loading on MIL-125-NH2 is very low due to small pore size and few amino groups on the surface of the MIL-125-NH2. In this work, catalase (CAT) was immobilized on the MIL-125-NH2 and amino functionalized MIL-125-NH2 by adsorption (CAT@MIL-125-NH2) and covalent binding (CAT@Amino MIL-125-NH2), respectively. Compared with the CAT@MIL-125-NH2 and free CAT, the CAT@Amino MIL-125-NH2 displayed high activity recovery, good pH stability, stability against denaturants, and thermostability. Furthermore, activity recovery of CAT@Amino MIL-125-NH2 was 56% higher than CAT@MIL-125-NH2. The CAT@Amino MIL-125-NH2 still retained 50% residual activity for 14 days at room temperature, whereas free CAT lost activity after storage for 1 day at the same storage conditions. Furthermore, the CAT@Amino MIL-125-NH2 maintained 62% of its initial activity after 4 consecutive uses, showing good reusability. The results showed that the amino functionalized MIL-125-NH2 is an excellent carrier of enzyme immobilization.
Similar content being viewed by others
References
Knowles, J. R. (1991) Enzyme catalysis: not different, just better. Nature. 350: 121–124.
Cantone, S., V. Ferrario, L. Corici, C. Ebert, D. Fattor, P. Spizzo, and L. Gardossi (2013) Efficient immobilisation of industrial biocatalysts: criteria and constraints for the selection of organic polymeric carriers and immobilisation methods. Chem. Soc. Rev. 42: 6262–6276.
Franssen, M. C. R., P. Steunenberg, E. L. Scott, H. Zuilhof, and J. P. M. Sanders (2013) Immobilised enzymes in biorenewables production. Chem. Soc. Rev. 42: 6491–6533.
Cui, J. and S. Jia (2015) Optimization protocols and improved strategies of cross-linked enzyme aggregates technology: current development and future challenges. Crit. Rev. Biotechnol. 35: 15–28.
Rodrigues, R. C., C. Ortiz, Á. Berenguer-Murcia, R. Torres, and R. Fernández-Lafuente (2013) Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 42: 6290–6307.
Adlercreutz, P. (2013) Immobilisation and application of lipases in organic media. Chem. Soc. Rev. 42: 6406–6436.
DiCosimo, R., J. McAuliffe, A. J. Poulose, and G. Bohlmann (2013) Industrial use of immobilized enzymes. Chem. Soc. Rev. 42: 6437–6474.
Hult, K. and P. Berglund (2007) Enzyme promiscuity: mechanism and applications. Trends Biotechnol. 25: 231–238.
Verma, M. L., M. Puri, and C. J. Barrow (2016) Recent trends in nanomaterials immobilised enzymes for biofuel production. Crit. Rev. Biotechnol. 36: 108–119.
Iyer, P. V. and L. Ananthanarayan (2008) Enzyme stability and stabilization—Aqueous and non-aqueous environment. Process Biochem. 43: 1019–1032.
Fernandez-Lafuente, R. (2009) Stabilization of multimeric enzymes: Strategies to prevent subunit dissociation. Enzyme Microb. Technol. 45: 405–408.
Mateo, C., J. M. Palomo, G. Fernandez-Lorente, J. M. Guisan, and R. Fernandez-Lafuente (2007) Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 40: 1451–1463.
Hernandez, K. and R. Fernandez-Lafuente (2011) Control of protein immobilization: coupling immobilization and site-directed mutagenesis to improve biocatalyst or biosensor performance. Enzyme Microb. Technol. 48: 107–122.
Garcia-Galan, C., Á. Berenguer-Murcia, R. Fernandez-Lafuente, and R. C. Rodrigues (2011) Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 353: 2885–2904.
Fernandez-Lopez, L., S. G. Pedrero, N. Lopez-Carrobles, B. C. Gorines, J. J. Virgen-Ortíz, and R. Fernandez-Lafuente (2017) Effect of protein load on stability of immobilized enzymes. Enzyme Microb. Technol. 98: 18–25.
Hwang, E. T. and M. B. Gu (2013) Enzyme stabilization by nano/microsized hybrid materials. Eng. Life Sci. 13: 49–61.
Barbosa, O., C. Ortiz, Á. Berenguer-Murcia, R. Torres, R. C. Rodrigues, and R. Fernandez-Lafuente (2015) Strategies for the one-step immobilization-purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 33: 435–456.
Sheldon, R. A. and S. van Pelt (2013) Enzyme immobilisation in biocatalysis: why, what and how. Chem. Soc. Rev. 42: 6223–6235.
Barbosa, O., R. Torres, C. Ortiz, A. Berenguer-Murcia, R. C. Rodrigues, and R. Fernandez-Lafuente (2013) Heterofunctional supports in enzyme immobilization: from traditional immobilization protocols to opportunities in tuning enzyme properties. Biomacromolecules. 14: 2433–2462.
Mohamad, N. R., N. H. C. Marzuki, N. A. Buang, F. Huyop, and R. A. Wahab (2015) An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 29: 205–220.
Lian, X., Y. Fang, E. Joseph, Q. Wang, J. Li, S. Banerjee, C. Lollar, X. Wang, and H. C. Zhou (2017) Enzyme-MOF (metalorganic framework) composites. Chem. Soc. Rev. 46: 3386–3401.
Sheldon, R. A. (2011) Characteristic features and biotechnological applications of cross-linked enzyme aggregates (CLEAs). Appl. Microbiol. Biotechnol. 92: 467–477.
Wang, M., W. Qi, R. Su, and Z. He (2015) Advances in carrier-bound and carrier-free immobilized nanobiocatalysts. Chem. Eng. Sci. 135: 21–32.
Cui, J., S. Ren, B. Sun, and S. Jia (2018) Optimization protocols and improved strategies for metal-organic frameworks for immobilizing enzymes: current development and future challenges. Coord. Chem. Rev. 370: 22–41.
Cui, J., Y. Zhao, Y. Feng, T. Lin, C. Zhong, Z. Tan, and S. Jia (2017) Encapsulation of spherical cross-linked phenylalanine ammonia lyase aggregates in mesoporous biosilica. J. Agric. Food Chem. 65: 618–625.
Wang, M., C. Jia, W. Qi, Q. Yu, X. Peng, R. Su, and Z. He (2011) Porous-CLEAs of papain: application to enzymatic hydrolysis of macromolecules. Bioresour. Technol. 102: 3541–3545.
Kumar, V. V., M. P. Prem Kumar, K. V. Thiruvenkadaravi, P. Baskaralingam, P. Senthil Kumar, and S. Sivanesan (2012) Preparation and characterization of porous cross linked laccase aggregates for the decolorization of triphenyl methane and reactive dyes. Bioresour. Technol. 119: 28–34.
Lopez-Gallego, F., T. Montes, M. Fuentes, N. Alonso, V. Grazu, L. Betancor, J. M. Guisán, and R. Fernández-Lafuente (2005) Improved stabilization of chemically aminated enzymes via multipoint covalent attachment on glyoxyl supports. J. Biotechnol. 116: 1–10.
Ansari, S. A. and Q. Husain (2012) Potential applications of enzymes immobilized on/in nano materials: A review. Biotechnol. Adv. 30: 512–523.
Kim, J., J. W. Grate, and P. Wang (2008) Nanobiocatalysis and its potential applications. Trends Biotechnol. 26: 639–646.
Deng, H., C. J. Doonan, H. Furukawa, R. B. Ferreira, J. Towne, C. B. Knobler, B. Wang, and O. M. Yaghi (2010) Multiple functional groups of varying ratios in metal-organic frameworks. Science. 327: 846–850.
Cao, S. L., D. M. Yue, X. H. Li, T. J. Smith, N. Li, M. H. Zong, H. Wu, Y. Z. Ma, and W. Y. Lou (2016) Novel nano-/micro-biocatalyst: soybean epoxide hydrolase immobilized on UiO-66-NH2 MOF for efficient biosynthesis of enantiopure (R)-1, 2-octanediol in deep eutectic solvents. ACS Sustainable Chem. Eng. 4: 3586–3595.
Sun, B., M. Bilal, S. Jia, Y. Jiang, and J. Cui (2019) Design and bio-applications of biological metal-organic frameworks. Korean J. Chem. Eng. 36: 1949–1964.
Hassanzadeh Fard, Z., N. E. Wong, C. D. Malliakas, P. Ramaswamy, J. M. Taylao, K. Otsubo, and G. K. H. Shimizu (2018) Superprotonic phase change to a robust phosphonate metal-organic framework. Chem. Mater. 30: 314–318.
Gu, Z., L. Chen, X. Li, L. Chen, Y. Zhang, and C. Duan (2019) NH2-MIL-125(Ti)-derived porous cages of titanium oxides to support Pt-Co alloys for chemoselective hydrogenation reactions. Chem. Sci. 10: 2111–2117.
Rengaraj, A., P. Puthiaraj, N. S. Heo, H. Lee, S. K. Hwang, S. Kwon, W. S. Ahn, and Y. S. Huh (2017) Porous NH2-MIL-125 as an efficient nano-platform for drug delivery, imaging, and ROS therapy utilized Low-Intensity Visible light exposure system. Colloids Surf. B. Biointerfaces. 160: 1–10.
Kim, S. N., J. Kim, H. Y. Kim, H. Y. Cho, and W. S. Ahn (2013) Adsorption/catalytic properties of MIL-125 and NH2-MIL-125. Cataly Today. 204: 85–93.
Guo, F., J. H. Guo, P. Wang, Y. S. Kang, Y. Liu, J. Zhao, and W. Y. Sun (2019) Facet-dependent photocatalytic hydrogen production of metal-organic framework NH2-MIL-125(Ti). Chem. Sci. 10: 4834–4838.
Jeremias, F., V. Lozan, S. K. Henninger, and C. Janiak (2013) Programming MOFs for water sorption: amino-functionalized MIL-125 and UiO-66 for heat transformation and heat storage applications. Dalton Trans. 42: 15967–15973.
Feng, Y., L. Zhong, Y. Hou, S. Jia, and J. Cui (2019) Acid-resistant enzyme@MOF nanocomposites with mesoporous silica shells for enzymatic applications in acidic environments. J. Biotechnol. 306: 54–61.
Kim, H. Y., S. N. Kim, J. Kim, and W. S. Ahn (2013) Liquid phase adsorption of selected chloroaromatic compounds over metal organic frameworks. Mater. Res. Bull. 48: 4499–4505.
Tüzmen, N., T. Kalburcu, and A. Denizli (2012) Immobilization of catalase via adsorption onto metal-chelated affinity cryogels. Process Biochem. 47: 26–33.
Cui, J., Y. Feng, and S. Jia (2018) Silica encapsulated catalase@metal-organic framework composite: a highly stable and recyclable biocatalyst. Chem. Eng. J. 351: 506–514.
Chen, C., W. Sun, H. Lv, H. Li, Y. Wang, and P. Wang (2018) Spacer arm-facilitated tethering of laccase on magnetic polydopamine nanoparticles for efficient biocatalytic water treatment. Chem. Eng. J. 350: 949–959.
Acknowledgments
This work is partially supported by the Science and Technology Program of Tianjin, China (project no. 20ZYJDJC00080), the Key Projects of Tianjin Natural Science Foundation, China (project no. 19JCZDJC38100), Key R & D projects in Zhongning County, China (project no. 2021YBYF0808), project of Guangxi Key Research and Development program, China (project no. GuikeAB21238005), and project of Key Research and Development of Shandong province, China (project no. 2021CXGC010509).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare no conflict of interest.
Neither ethical approval nor informed consent was required for this study.
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Z., Liu, Y., Li, J. et al. Efficient Immobilization of Enzymes on Amino Functionalized MIL-125-NH2 Metal Organic Framework. Biotechnol Bioproc E 27, 135–144 (2022). https://doi.org/10.1007/s12257-020-0393-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-020-0393-y