Abstract
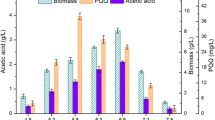
The effects of different oxygen uptake rates (OUR) on the physiological metabolism of Rhodobacter sphaeroides were investigated systematically in 50 L fermenters due to the significant influence on industrial coenzyme Q10 production under oxygen supply limitation. Meanwhile, the seriously decreased oxygen transfer rate caused by the increased broth viscosity was successfully prevented with OUR-directed continuous ammonium sulfate feeding in the late fermentation phase. The statistical analysis results showed that controlling OUR constantly at 45 ± 2.2 mmol/L/h by the oxygen supply level adjustment and the continuous ammonium sulfate feeding could greatly enhance Q10 production. This OUR-Stat controlling strategy successfully achieved the maximal coenzyme Q10 production (2584 ± 82 mg/L), which was 15.4% higher than that of the control. The highest specific CoQ10 content was 25.9 mg/(g DCW)), and the yield of CoQ10 to glucose consumption was up to 19.37 mg/g. These results demonstrated that the optimal OUR-Stat controlling strategy would be effective and economical for improving the industrial CoQ10 production.
Similar content being viewed by others
References
Cluis, C. P., A. M. Burja, and V. J. J. Martin (2007) Current prospects for the production of coenzyme Q10 in microbes. Trends Biotechnol. 25: 514–521.
Zhu, Z. G., M. X. Sun, W. L. Zhang, W. W. Wang, Y. M. Jin, and C. L. Xie (2017) The efficacy and safety of coenzyme Q10 in Parkinson’s disease: a meta-analysis of randomized controlled trials. Neurol. Sci. 38: 215–224.
Park, Y. C., S. J. Kim, J. H. Choi, W. H. Lee, K. M. Park, M. Kawamukai, Y. W. Ryu, and J. H. Seo (2005) Batch and fed-batch production of coenzyme Q10 in recombinant Escherichia coli containing the decaprenyl diphosphate synthase gene from Gluconobacter suboxydans. Appl. Microbiol. Biotechnol. 67: 192–196.
Nasoohi, S., L. Simani, F. Khodagholi, S. Nikseresht, M. Faizi, and N. Naderi (2019) Coenzyme Q10 supplementation improves acute outcomes of stroke in rats pretreated with atorvastatin. Nutr. Neurosci. 22: 264–272.
Jeya, M., H. J. Moon, J. L. Lee, I. W. Kim, and J. K. Lee (2010) Current state of coenzyme Q10 production and its applications. Appl. Microbiol. Biotechnol. 85: 1653–1663.
Cluis, C. P., D. Pinel, and V. J. Martin (2012) The production of coenzyme Q10 in microorganisms. Subcell. Biochem. 64: 303–326.
Rivara, M. B., C. K. Yeung, C. Robinson-Cohen, B. R. Phillips, J. Ruzinski, D. Rock, L. Linke, D. D. Shen, T. A. Ikizler, and J. Himmelfarb (2017) Effect of coenzyme Q10 on biomarkers of oxidative stress and cardiac function in hemodialysis patients: the coq10 biomarker trial. Am. J. Kidney Dis. 69: 389–399.
Negida, A., A. Menshawy, G. El Ashal, Y. Elfouly, Y. Hani, Y. Hegazy, S. El Ghonimy, S. Fouda, and Y. Rashad (2016) Coenzyme Q10 for patients with parkinson’s disease: a systematic review and meta-analysis. CNS Neurol. Disord. Drug Targets. 15: 45–53.
Shults, C. W., D. Oakes, K. Kieburtz, M. F. Beal, R. Haas, S. Plumb, J. L. Juncos, J. Nutt, I. Shoulson, J. Carter, K. Kompoliti, J. S. Perlmutter, S. Reich, M. Stern, R. L. Watts, R. Kurlan, E. Molho, M. Harrison, and M. Lew (2002) Effects of coenzyme Q10 in early parkinson disease: evidence of slowing of the functional decline. Arch. Neurol. 59: 1541–1550.
Xu, Z., J. Huo, X. Ding, M. Yang, L. Li, J. Dai, K. Hosoe, H. Kubo, M. Mori, K. Higuchi, and J. Sawashita (2017) Coenzyme Q10 Improves lipid metabolism and ameliorates obesity by regulating CaMKII-mediated PDE4 inhibition. Sci. Rep. 7: 8253.
Sarmiento, A., J. Diaz-Castro, M. Pulido-Moran, N. Kajarabille, R. Guisado, and J. J. Ochoa (2016) Coenzyme Q10 supplementation and exercise in healthy humans: a systematic review. Curr. Drug Metab. 17: 345–358.
de Dieu Ndikubwimana, J. and B. H. Lee (2014) Enhanced production techniques, properties and uses of coenzyme Q10. Biotechnol. Lett. 36: 1917–1926.
Parmar, S. S., A. Jaiwal, O. P. Dhankher, and P. K. Jaiwal (2015) Coenzyme Q10 production in plants: current status and future prospects. Crit. Rev. Biotechnol. 35: 152–164.
Tian, Y., T. Yue, Y. Yuan, P. K. Soma, and Y. M. Lo (2010) Improvement of cultivation medium for enhanced production of coenzyme Q10 by photosynthetic Rhodospirillum rubrum. Biochem. Eng. J. 51: 160–166.
Dai, G., L. Miao, T. Sun, Q. Li, D. Xiao, and X. Zhang (2015) Production of coenzyme Q10 by metabolically engineered Escherichia coli. Sheng Wu Gong Cheng Xue Bao. 31: 206–219.
Qiu, L., W. Wang, W. Zhong, L. Zhong, J. Fang, X. Li, S. Wu, and J. Chen (2011) Coenzyme Q10 production by Sphingomonas sp. ZUTE03 with novel precursors isolated from tobacco waste in a two-phase conversion system. J. Microbiol. Biotechnol. 21: 494–502.
Kawamukai, M. (2002) Biosynthesis, bioproduction and novel roles of ubiquinone. J. Biosci. Bioeng. 94: 511–517.
Choi, J. H., Y. W. Ryu, and J. H. Seo (2005) Biotechnological production and applications of coenzyme Q10. Appl. Microbiol. Biotechnol. 68: 9–15.
Lu, W., L. Ye, X. Lv, W. Xie, J. Gu, Z. Chen, Y. Zhu, A. Li, and H. Yu (2015) Identification and elimination of metabolic bottlenecks in the quinone modification pathway for enhanced coenzyme Q10 production in Rhodobacter sphaeroides.Metab. Eng. 29: 208–216.
Zhu, Y., L. Ye, Z. Chen, W. Hu, Y. Shi, J. Chen, C. Wang, Y. Li, W. Li, and H. Yu (2017) Synergic regulation of redox potential and oxygen uptake to enhance production of coenzyme Q10 in Rhodobacter sphaeroides.Enzyme Microb. Technol. 101: 36–43.
Yuan, Y., Y. Tian, and T. Yue (2012) Improvement of coenzyme Q10 production: Mutagenesis induced by high hydrostatic pressure treatment and optimization of fermentation conditions. J. Biomed. Biotechnol. 2: 607329.
Kien, N. B., I. S. Kong, M. G. Lee, and J. K. Kim (2010) Coenzyme Q10 production in a 150-l reactor by a mutant strain Ofrhodobacter sphaeroides. J. Ind. Microbiol. Biot. 37: 521–529.
Sakato, K., H. Tanaka, and S. Shibata (1992) Agitation-aeration studies on coenzyme Q10 production using Rhodopseudomonas spheroides. Biotechnol. Appl. Biochem. 16: 19–28.
Bae, Y. H., D. H. Kweon, Y. C. Park, and J. H. Seo (2014) Deletion of the hxk2 gene in Saccharomyces cerevisiae enables mixed sugar fermentation of glucose and galactose in oxygen-limited conditions. Process Biochem. 49: 547–553.
Johnsson, O., J. Andersson, G. Lidén, C. Johnsson, and T. Gglund (2015) Modelling of the oxygen level response to feed rate perturbations in an industrial scale fermentation process. Process Biochem. 50: 507–516.
Reis, A., F. Raquel, R. Torres, F. Leite, M. Patrícia, and E. Vidal (2014) Oxygen-limited cellobiose fermentation and the characterization of the cellobiase of an industrial dekkera/brettanomyces bruxellensis strain. SpringerPlus. 3: 38–42.
Yegneswaran, P. K., M. R. Gray, and B. G. Thompson (1991) Effect of dissolved oxygen control on growth and antibiotic production in Streptomyces clavuligerus fermentations. Biotechnol. Prog. 7: 246–250.
Wang, Z. J., H. Y. Wang, Y. Li, J. Chu, M. Huang, and Y. Zhuang (2010) Improved vitamin B12 production by step-wise reduction of oxygen uptake rate under dissolved oxygen limiting level during fermentation process. Bioresour. Technol. 101: 2845–2852.
Zhang, S., J. Chu, and Y. Zhuang (2004) A multi-scale study of industrial fermentation processes and their optimization. Adv. Biochem. Eng. Biotechnol. 87: 97–150.
Palomares, L. A., S. Lopez, and O. T. Ramirez (2004) Utilization of oxygen uptake rate to assess the role of glucose and glutamine in the metabolism of infected insect cell cultures. Biochem. Eng. J. 19: 87–93.
Feng, Q., L. Mi, L. Li, R. Liu, L. Xie, and H. Tang (2006) Application of “oxygen uptake rate-amino acids” associated mode in controlled-fed perfusion culture. J. Biotechnol. 122: 422–30.
Schafer, S., J. Schrader, and D. Sell (2004) Oxygen uptake rate measurements to monitor the activity of terpene transforming fungi. Process Biochem. 39: 2221–2228.
Wang, Y., J. Chu, Y. Zhuang, Y. Wang, J. Xia, and S. Zhang (2009) Industrial bioprocess control and optimization in the context of systems biotechnology. Biotechnol. Adv. 27: 989–995.
Ha, S. J., S. Y. Kim, J. H. Seo, M. Jeya, Y. W. Zhang, and T. Ramu (2009) Ca2+ increases the specific coenzyme Q10 content in Agrobacterium tumefaciens. Bioprocess Biosyst. Eng. 32: 697–700.
Zhang, L., L. S. Liu, K. F. Wang, L. Xu, L. M. Zhou, W. S. Wang, C. Li, Z. Xu, T. Shi, H. H. Chen, Y. H. Li, H. Xu, X. L. Yang, Z. C. Zhu, B. Q. Chen, D. Li, G. H. Zhan, S. L. Zhang, L. X. Zhang, and G. Y. Tan (2019) Phosphate limitation increases coenzyme Q10 production in industrial Rhodobacter sphaeroides HY01. Synth. Syst. Biotechnol. 4: 212–219.
Maskow, T., I. Anita, J. Yao, and H. Harms (2008) Observation of non-linear biomass-capacitance correlations: reasons and implications for bioprocess control. Biosens. Bioelectron. 24: 123–128.
Kanda, J. (1995) Determination of ammonium in seawater based on the indophenol reaction with o-phenylphenol (OPP). Water Res. 29: 2746–2750.
Chen, Y., Z. Wang, J. Chu, Y. Zhuang, S. Zhang, and X. Yu (2013) Significant decrease of broth viscosity and glucose consumption in erythromycin fermentation by dynamic regulation of ammonium sulfate and phosphate. Bioresour. Technol. 134: 173–179.
Liang, J. and J. Yuan (2007) Oxygen transfer model in recombinant Pichia pastoris and its application in biomass estimation. Biotechnol. Lett. 29: 27–35.
Zou, X., H. F. Hang, J. Chu, Y. P. Zhuang, and S. L. Zhang (2009) Oxygen uptake rate optimization with nitrogen regulation for erythromycin production and scale-up from 50 L to 372 m3 scale. Bioresour. Technol. 100: 1406–1412.
Bandaiphet, C. and P. Prasertsan (2006) Effect of aeration and agitation rates and scale-up on oxygen transfer coefficient, kLa in exopolysaccharide production from Enterobacter cloacae WD7. Carbohydr. Polym. 66: 216–228.
Xia, J. Y., Y. H. Wang, S. L. Zhang, N. Chen, P. Yin, and Y. P. Zhuang (2009) Fluid dynamics investigation of variant impeller combinations by simulation and fermentation experiment. Biochem. Eng. J. 43: 252–260.
Mahadevan, S., B. Dhandapani, S. Sivaprakasam, and A. B. Mandal (2010) Batch kinetic studies on growth of salt tolerant Pseudomonas aeruginosase creting protease in a biocalorimeter. Biotechnol. Bioprocess Eng. 15: 670–675.
Dallner, G. and P. J. Sindelar (2000) Regulation of ubiquinone metabolism. Free Radic. Biol. Med. 29: 285–294.
Robertson, D., B. A. McCormack, and G. P. Bolwell (1995) Cell wall polysaccharide biosynthesis and related metabolism in elicitor-stressed cells of French bean (Phaseolus vulgaris L.). Biochem. J. 306: 745–750.
Franken, J., B. A. Brandt, S. L. Tai, and F. F. Bauer (2013) Biosynthesis of levan, a bacterial extracellular polysaccharide, in the yeast Saccharomyces cerevisiae. PLoS One. 8: e77499.
Mulchandani, A., J. H. Luong, and A. Leduy (1988) Batch kinetics of microbial polysaccharide biosynthesis. Biotechnol. Bioeng. 32: 639–646.
Kawamukai, M. (2002) Biosynthesis, bioproduction and novel roles of ubiquinone. J. Biosci. Bioeng. 94: 511–517.
Lu, W., L. Ye, H. Xu, W. Xie, and H. Yu (2014) Enhanced production of coenzyme Q10 by self-regulating the engineered MEP pathway in Rhodobacter sphaeroides.Biotechnol. Bioeng. 111: 761–769.
Ha, S. J., S. Y. Kim, J. H. Seo, H. J. Moon, K. M. Lee, and J. K. Lee (2007) Controlling the sucrose concentration increases Coenzyme Q10 production in fed-batch culture of Agrobacterium tumefaciens. Appl. Microbiol. Biotechnol. 76: 109–116.
Merten, O. W., G. E. Palfi, and J. Steiner (1986) On-line determination of biochemical/physiological parameters in the fermentation of animal cells in a continuous or discontinuous mode. Adv. Biotechnol. Processes. 6: 111–178.
Acknowledgements
This work was financially supported by the National key research and development program (Grant No. 2017YFF0204602), the Fundamental research funds for the central universities (No. 222201714024), and innovation and intelligence 111 plan.
The authors declare no conflict of interest.
Neither ethical approval nor informed consent was required for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, ZJ., Zhang, X., Wang, P. et al. Oxygen Uptake Rate Controlling Strategy Balanced with Oxygen Supply for Improving Coenzyme Q10 Production by Rhodobacter sphaeroides. Biotechnol Bioproc E 25, 459–469 (2020). https://doi.org/10.1007/s12257-019-0461-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-019-0461-3