Abstract
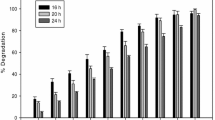
In the hydrolysis of microalgae with a solid catalyst, a novel rotating packed bed reactor (RPBR) was developed to improve the recyclability of the Amberlyst 36, a solid-acid catalyst. In a previous study, the hydrolysis of Golenkinia sp. using Amberlyst 36 with nitric acid in a conventional batch reactor was carried out, but the solid catalyst could only be recycled for four consecutive runs due to damage to the catalyst structure. As a solution to this catalyst degradation/destruction problem, Amberlyst 36 was charged in the packed bed column and was rotated instead of using impellers in an autoclave reactor. The packed bed column prevented the solid-acid catalyst from physical attrition caused by stirring with impellers, resultantly the recyclability of the solid catalyst increased dramatically. In addition to this, our approach of using the rotating packed bed column facilitated the particle-particle interactions between the catalyst and biomass, as a result of which high sugar yields of more than 70% (g/g) was achieved within four runs. Moreover, the reusability of Amberlyst 36 could be raised to 11 runs by simply employing a combination of two additional processes, sonication and reactivation processes with sulfuric acid solution. With our novel approach of using RPBR, a solid catalyst can be used perpetually for hydrolysis of algal biomass, which makes the process of hydrolysis efficient and economical compare to the conventional batch reactor.
Similar content being viewed by others
References
Fozer, D., N. Valentinyi, L. Racz, and P. Mizsey (2017) Evaluation of microalgae-based biorefinery alternatives. Clean Technol. Environ. Policy. 19: 501–515.
Vanthoor-Koopmans, M., R. H. Wijffels, M. J. Barbosa, and M. H. Eppink (2013) Biorefinery of microalgae for food and fuel. Bioresour. Technol. 135: 142–149.
Chen, C. Y., X. Q. Zhao, H. W. Yen, S. H. Ho, C. L. Cheng, D. J. Lee, F. W. Bai, and J. S. Chang (2013) Microalgae-based carbohydrates for biofuel production. Biochem. Eng. J. 78: 1–10.
Yen, H. W., I. C. Hu, C. Y. Chen, S. H. Ho, D. J. Lee, and J. S. Chang (2013) Microalgae-based biorefinery—from biofuels to natural products. Bioresour. Technol. 135: 166–174.
Brown, M. R. (1991) The amino-acid and sugar composition of 16 species of microalgae used in mariculture. J. Exp. Mar. Biol. Ecol. 145: 79–99.
Kim, K. H., I. S. Choi, H. M. Kim, S. G. Wi, and H. J. Bae (2014) Bioethanol production from the nutrient stress-induced microalga Chlorella vulgaris by enzymatic hydrolysis and immobilized yeast fermentation. Bioresour. Technol. 153: 47–54.
Hernandez, D., B. Riano, M. Coca, and M. C. Garcia-Gonzalez (2015) Saccharification of carbohydrates in microalgal biomass by physical, chemical and enzymatic pre-treatments as a previous step for bioethanol production. Chem. Eng. J. 262: 939–945.
Choi, S. P., M. T. Nguyen, and S. J. Sim (2010) Enzymatic pretreatment of Chlamydomonas reinhardtii biomass for ethanol production. Bioresour. Technol. 101: 5330–5336.
Ho, S. H., S. W. Huang, C. Y. Chen, T. Hasunuma, A. Kondo, and J. S. Chang (2013) Bioethanol production, using carbohydraterich microalgae biomass as feedstock. Bioresour. Technol. 135: 191–198.
Peng, H., L. Zhai, S. Xu, P. Xu, C. He, Y. Xiao, and Y. Gao (2018) Efficient hydrolysis of raw microalgae starch by an alpha-amylase (AmyP) of glycoside hydrolase subfamily GH13_37. J. Agric. Food Chem. 66: 12748–12755.
Sedighi, M., H. Jalili, M. Darvish, Sadeghi, and S. O. Ranaei-Siadat (2019) Enzymatic hydrolysis of microalgae proteins using serine proteases: A study to characterize kinetic parameters. Food Chem. 284: 334–339.
Wang, X., X. Liu, and G. Wang (2011) Two-stage hydrolysis of invasive algal feedstock for ethanol fermentation. J. Integr. Plant Biol. 53: 246–252.
Harun, R. and M. K. Danquah (2011) Influence of acid pre-treatment on microalgal biomass for bioethanol production. Process Biochem. 46: 304–309.
Nguyen, M. T., S. P. Choi, J. Lee, J. H. Lee, and S. J. Sim (2009) Hydrothermal acid pretreatment of Chlamydomonas reinhardtii biomass for ethanol production. J. Microbiol. Biotechn. 19: 161–166.
Choi, S. A., W. I. Choi, J. S. Lee, S. W. Kim, G. A. Lee, J. Yun, and J. Y. Park (2015) Hydrothermal acid treatment for sugar extraction from Golenkinia sp. Bioresour. Technol. 190: 408–411.
de Farias Silva, C. E. and A. Bertucco (2017) Dilute acid hydrolysis of microalgal biomass for bioethanol production: an accurate kinetic model of biomass solubilization, sugars hydrolysis and nitrogen/ash balance. Reac. Kinet. Mech. Cat. 122: 1095–1114.
Fu, Q., H. Zhang, H. Chen, Q. Liao, A. Xia, Y. Huang, X. Zhu, A. Reungsang, and Z. Liu (2018) Hydrothermal hydrolysis pretreatment of microalgae slurries in a continuous reactor under subcritical conditions for large-scale application. Bioresour. Technol. 266: 306–314.
Joo, H. W., Y. J. Kim, J. Park, and Y. K. Chang (2017) Hydrolysis of Golenkinia sp biomass using Amberlyst 36 and nitric acid as catalysts. Algal Res. 25: 32–38.
Ramshaw, C. (1983) Higee distillation - an example of process intensification. Chem. Eng.-London. 389: 13–14.
Arshadi, M., M. K. Abdolmaleki, F. Mousavinia, A. Khalafi-Nezhad, H. Firouzabadi, and A. Gil (2016) Degradation of methyl orange by heterogeneous Fenton-like oxidation on a nano-organometallic compound in the presence of multi-walled carbon nanotubes. Chem. Eng. Res. Des. 112: 113–121.
Chen, Y. H., C. Y. Chang, W. L. Su, C. C. Chen, C. Y. Chiu, Y. H. Yu, P. C. Chiang, and S. I. M. Chiang (2004) Modeling ozone contacting process in a rotating packed bed. Ind. Eng. Chem. Res. 43: 228–236.
Lin, C. C., W. T. Liu, and C. S. Tan (2003) Removal of carbon dioxide by absorption in a rotating packed bed. Ind. Eng. Chem. Res. 42: 2381–2386.
Kelleher, T. and J. R. Fair (1996) Distillation studies in a high-gravity contactor. Ind. Eng. Chem. Res. 35: 4646–4655.
Chen, Y. H., Y. H. Huang, R. H. Lin, and N. C. Shang (2010) A continuous-flow biodiesel production process using a rotating packed bed. Bioresour. Technol. 101: 668–673.
Chen, Y. H., Y. H. Huang, R. H. Lin, N. C. Shang, C. Y. Chang, C. C. Chang, P. C. Chiang, and C. Y. Hu (2011) Biodiesel production in a rotating packed bed using K/gamma-Al2O3 solid catalyst. J. Taiwan Inst. Chem. Eng. 42: 937–944.
Oh, S. J., J. Park, J. G. Na, Y. K. Oh, and Y. K. Chang (2015) Production of 5-hydroxymethylfurfural from agarose by using a solid acid catalyst in dimethyl sulfoxide. Rsc. Adv. 5: 47983–47989.
Sampath, G. and S. Kannan (2013) Fructose dehydration to 5-hydroxymethylfurfural: Remarkable solvent influence on recyclability of Amberlyst-15 catalyst and regeneration studies. Catal. Commun. 37: 41–44.
Acknowledgements
This work was supported by the Advanced Biomass R&D Center (ABC) of the Global Frontier Project funded by the Ministry of Science and ICT. (ABC-2010-0029728).
Author information
Authors and Affiliations
Contributions
Hyun Woo Joo, Hoyoung Ryu, and Yong Keun Chang were involved in the design and perform of the experiment, to the analysis of the results, and to the writing and revising of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest Statement There is no conflict of interest statement
Statement of Informed Consent, Human/animal Rights No conflicts, informed consent, human or animal rights applicable.
Declaration of Authors Agreement to Authorship and Submission of the Manuscript for Peer Review All the authors confirmed the manuscript authorship and agreed to submit it for peer review.
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Joo, H.W., Ryu, H. & Chang, Y.K. Hydrolysis of Golenkinia sp. by Using a Rotating Packed Bed Reactor and Regeneration of Solid Acid Catalyst. Biotechnol Bioproc E 24, 990–996 (2019). https://doi.org/10.1007/s12257-019-0417-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-019-0417-7