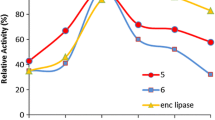
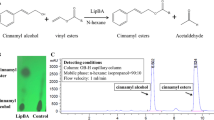
Abstract
Capsaicin analogues are typically synthetized by condensation of the amide group of vanillylamine with a fatty acid derivative. The enzyme of choice to perform this reaction is Candida antarctica lipase B; however, this enzyme is unable to react with the phenolic substiruents of the vanillyl ring. So far, this can only be achieved chemically, resulting in capsaicin esters with higher lipophilicity and less irritation than the parent compound. In need of searching for new capsaicin derivatives, we investigated the transesterification of the phenolic OH group of capsaicin and several capsaicin analogues by Candida antarctica lipase A (CALA) with vinyl esters. Capsaicin esters were successfully synthetized with a reaction yield of 80.6% and 57.5% with vinyl butyrate and vinyl laurate, respectively. When the reactions were performed with capsaicin analogues of different acyl chain lengths, CALA exhibited a noticeable transesterification preference for medium-length capsaicin analogues. In an attempt to explore the catalytic limits of CALA, we performed the transesterification of capsaicin from Capsicum oleoresin. The results showed similar transesterification yields to those obtained with semi-pure capsaicin. Within our knowledge, this is the first report to achieve the synthesis of phenolic OH esters of capsaicin and capsaicin analogues by enzymatic means.
Similar content being viewed by others
References
Caterina, M. J., M. A. Schumacher, M. Tominaga, T. A. Rosen, J. D. Levine, and D. Julius (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 389: 816–824.
Balabathula, P., H. Bhattacharjee, L. A. Thoma, R. J. Nolly, F. P. Horton, G. D. Stornes, J. Y. Wan, I. M. Brooks, G. A. Bachmann, D. C. Foster, and C. S. Brown (2014) Potency and stability of intradermal capsaicin: implications for use as a human model of pain in multicenter clinical trials. Clin. Exp. Pharmacol. 4: 142.
Nilius, B. and R. Vennekens (2010) TRP channels and human disease, pp. 1–67. In: A. Gomtsyan and C. R. Faltynek (eds.). Vanilloid receptor TRPV1 in drug discovery: targeting pain and other pathological disorders. John Wiley & Sons, Inc., Hoboken, NJ, USA.
Dickenson, A. H. and A. Dray (1991) Selective antagonism of capsaicin by capsazepine: evidence for a spinal receptor site in capsaicin-induced antinociception. Br. J. Pharmacol. 104: 1045–1049.
Urban, L. and A. Dray (1991) Capsazepine, a novel capsaicin antagonist, selectively antagonises the effects of capsaicin in the mouse spinal cord in vitro. Neurosci. Lett. 134: 9–11.
Walpole, C. S. J., S. Bevan, G. Bovermannm, J. J. Boelsterli, R. Breckenridge, J. W. Davies, G. A. Hughes, I. James, L. Oberer, J. Winter, and R. Wrigglesworth (1994) The discovery of capsazepine, the first competitive antagonist of the sensory neuron excitants capsaicin and resiniferatoxin. J. Med. Chem. 37: 1942–1954.
Bevan, S., S. Hothi, G. Hughes, I. F. James, H. P. Rang, K. Shah, C. S. Walpole, and J. C. Yeats (1992) Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br. J. Pharmacol. 107: 544–552.
Appendino, G., S. Harrison, L. De Petrocellis, N. Daddario, F. Bianchi, A. Schiano Monello, M. Trevisani, F. Benvenuti, P. Geppetti, and V. Di Marzo (2003) Halogenation of a capsaicin analogue leads to novel vanilloid TRPVI receptor antagonists. Br. J. Pharmacol. 139: 1417–1424.
Appendino, G., N. Daddario, A. Minassi, A. S. Monello, L. De Petrocellis, and V. Di Marzo (2005) The taming of capsaicin. Reversal of the vanilloid activity of N-acylvanillamines by aromatic iodination. J. Med. Chem. 48: 4663–4669.
Walpole, C. S. J., S. Bevan, G. Bloomfield, R. Breckenridge, I. F. James, T. Ritchie, A. Szallasi, J. Winter, and R. Wrigglesworth (1996) Similarities and differences in the structure-activity relationships of capsaicin and resiniferatoxin analogues. J. Med. Chem. 39: 2939–2952.
Walpole, C. S. J., R. Wrigglesworth, S. Bevan, E. A. Campbell, A. Dray, I. F. James, M. N. Perkins, D. J. Reid, and J. Winter (1993) Analogs of capsaicin with agonist activity as novel analgesic agents; structure-activity studies. 1. The aromatic “A-region.” J. Med. Chem. 36: 2362–2372.
Singh, C. U. and R. N. Jagaveerabhadra (2011) Esters of capsaicin for treating pain. US Patent 7, 943, 666B2.
Reyes-Duarte, D., E. Castillo, R. Martinez, and A. López-Munguía (2002) Lipase-catalysed synthesis of olvanil in organic solvents. Biotechnol. Lett. 24: 2057–2061.
Castillo, E., A. Torres-Gavilán, P. Severiano, N. Arturo, and A. López-Munguia (2007) Lipase-catalyzed synthesis of pungent capsaicin analogues. Food Chem. 100: 1202–1208.
Castillo, E., I. López-Gonzalez, R. De Regil-Hernández, D. Reyes-Duarte, D. Sánchez-Herrera, A. López-Munguía, and A. Darszon (2007) Enzymatic synthesis of capsaicin analogs and their effect on the T-type Ca2+ channels. Biochem. Biophys. Res. Commun. 356: 424–430.
Wang, B., F. Yang, Y. F. Shan, W. W. Qiu, and J. Tang (2009) Highly efficient synthesis of capsaicin analogues by condensation of vanillylamine and acyl chlorides in a biphase H2O/CHCl3 system. Tetrahedron. 65: 5409–5412.
Kobata, K., M. Kobayashi, Y. Tamura, S. Miyoshi, S. Ogawa, and T. Watanabe (1999) Lipase-catalyzed synthesis of capsaicin analogs by transacylation of capsaicin with natural oils or fatty acid derivatives in n-hexane. Biotechnol. Lett. 21: 547–550.
Kobata, K., M. Kawamura, M. Toyoshima, Y. Tamura, S. Ogawa, and T. Watanabe (1998) Lipase-catalyzed synthesis of capsaicin analogs by amidation of vanillylamine with fatty acid derivatives. Biotechnol. Lett. 20: 451–454.
Cha, H. J., J. B. Park, and S. Park (2019) Esterification of secondary alcohols and multi-hydroxyl compounds by Candida antarctica lipase B and subtilisin. Biotechnol. Bioprocess Eng. 24: 41–47.
Chen, H., X. Meng, X. Xu, W. Liu, and S. Li (2018) The molecular basis for lipase stereoselectivity. Appl. Microbiol. Biotechnol. 102: 3487–3495.
Guyot, B., B. Bosquette, M. Pina, and J. Graille (1997) Esterification of phenolic acids from green coffee with an immobilized lipase from Candida antarctica in solvent-free medium. Biotechnol. Lett. 19: 529–532.
Figueroa-Espinoza, M. C., M. Laguerre, P. Villeneuve, and J. Lecomte (2013) From phenolics to phenolipids: optimizing antioxidants in lipid dispersions. Lipid Technol. 25: 131–134.
Sheldon, R. A. and S. van Pelt (2013) Enzyme immobilisation in biocatalysis: why, what and how. Chem Soc Rev. 42: 6223–6235.
Wohlgemuth, R. (2010) Biocatalysis — key to sustainable industrial chemistry. Curr Opin. Biotechnol. 21: 713–724.
Dominguez De Maria, P., C. Carboni-Oerlemans, B. Tuin, G. Bargeman, A. Van Der Meer, and R. Van Gemert (2005) Biotechnological applications of Candida antarctica lipase A: State-of-the-art. J. Mol. Catal. B Enzym. 37: 36–46.
Pihko, A. J., K. Lundell, L. Kanerva, and A. M. P. Koskinen (2004) Enantioselective synthesis of a hindered furyl substituted allyl alcohol intermediate: a case study in asymmetric synthesis. Tetrahedron. Asymmetry. 15: 1637–1643.
Skjot, M., L. De Maria, R. Chatterjee, A. Svendsen, S. A. Patkar, P. R. Ostergaard, and J. Brask (2009) Understanding the plasticity of the alpha/beta hydrolase fold: lid swapping on the Candida antarctica lipase B results in chimeras with interesting biocatalytic properties. Chembiochem. 10: 520–527.
From, M., P. Adlercreutz, and B. Mattiasson (1997) Lipase catalyzed esterification of lactic acid. Biotechnol. Lett. 19: 315–318.
Kim, H., Y. K. Choi, J. Lee, E. Lee, J. Park, and M. J. Kim (2011) Ionic-surfactant-coated Burkholderia cepacia lipase as a highly active and enantioselective catalyst for the dynamic kinetic resolution of secondary alcohols. Angew. Chem. Int. Ed. Engl. 50: 10944–10948.
Ema, T., M. Jittani, T. Sakai, and M. Utaka (1998) Lipase-catalyzed kinetic resolution of large secondary alcohols having tetraphenylporphyrin. Tetrahedron. Lett. 39: 6311–6314.
Ericsson, D. J., A. Kasrayan, P. Johansson, T. Bergfors, A. G. Sandström, J. E. Bäckvall, and S. L. Mowbray (2008) X-ray structure of Candida antarctica lipase A shows a novel lid structure and a likely mode of interfacial activation. J. Mol. Biol. 376: 109–119.
Seo, J. H., S. M. Lee, J. Lee, and J. B. Park (2015) Adding value to plant oils and fatty acids: biological transformation of fatty acids into co-hydroxycarboxylic, α,ω-dicarboxylic, and ω-aminocarboxylic acids. J. Biotechnol. 216: 158–166.
Lee, D., J. W. Song, M. Voß, E. Schuiten, R. K. Akula, Y. U. Kwon, U. Bornscheuer, and J. B. Park (2019) Enzyme cascade reactions for the biosynthesis of long chain aliphatic amines from renewable fatty acids. Adv. Synth. Catal. 361: 1359–1367.
Woo, J. M., E. Y. Jeon, E. J. Seo, J. H. Seo, D. Y. Lee, Y. J. Yeon, and J. B. Park (2018) Improving catalytic activity of the Baeyer-Vdliger monooxygenase-based Escherichia coli biocatalysts for the overproduction of (Z)-11-(heptanoyloxy)undec-9-enoic acid from ricinoleic acid. Sci. Rep. 8: 10280.
Seo, E. J., Y. J. Yeon, J. H. Seo, J. H. Lee, J. P. Bongol, Y. Oh, J. M. Park, S. M. Lim, C. G. Lee, and J. B. Park (2018) Enzyme/ whole-cell biotransformation of plant oils, yeast derived oils, and microalgae fatty acid methyl esters into n-nonanoic acid, 9-hydroxynonanoic acid, and 1,9-nonanedioic acid. Bioresour. Technol. 251: 288–294.
Cha, H. J., E. J. Seo, J. W. Song, H. J. Jo, A. R. Kumar, and J. B. Park (2018) Simultaneous enzyme/whole-cell biotransformation of C (18). ricinoleic acid into (R)-3-hydroxynonanoic acid, 9-hydroxynonanoic acid, and 1,9-nonanedioic acid 360: 696–703.
Jeon, E. Y., J. H. Seo, W. R. Kang, M. J. Kim, J. H. Lee, D. K. Oh, and J. B. Park (2016) Simultaneous enzyme/whole-cell biotransformation of plant oils into C(9). carboxylic acids 6: 7547–7553.
Ganesa, S., A. Karthik, S. B. Karnath, K. Prabahar, A. Ranjithkumar, S. Pathak, and N. Udupa (2008) Stability-indicating HPTLC determination of capsaicin in the bulk drug. J. Planar. Chromatogr. Mod. TLC 21: 271–275.
Singh, C. U. and R. N. Jagaveerabhadra (2009) Esters of capsaicinoids as dietary supplements. US Patent 20100120912A1.
Singh, C. U., D. L. Woody, and R. N. Jagaveerabhadra (2013) Pharmaceutical compositions comprising capsaicin esters for treating pain and cold sores. US Patent 20140134261.
Annesto, N., M. Ferrerò, S. Fernández, and V. Gotor (2002) Regioselective enzymatic acylation of methyl shikimate. Influence of acyl chain length and solvent polarity on enzyme specificity. J. Org. Chem. 67: 4978–4981.
Ishihara, K., S. I. Kwon, N. Masuoka, N. Nakajima, and H. Hamada (2010) One-procedure synthesis of capsiate from capsaicin by lipase-catalyzed dynamic transacylation. World J. Microbiol. Biotechnol. 26: 1337–1340.
Wrigglesworth, R., C. S. J. Walpole, S. Bevan, E. A. Campbell, A. Dray, G. A. Hughes, I. James, K. J. Masdin, and J. Winter (1996) Analogues of capsaicin with agonist activity as novel analgesic agents; structure-activity studies. 4. Potent, orally active analgesics. J. Med. Chem. 39: 4942–4951.
Mercadante, A. Z., D. B. Rodrigues, F. C. Perry, and L. R. B. Mariutti (2017) Carotenoid esters in foods - A review and practical directions on analysis and occurrence. Food Res. Int. 99: 830–850.
Minguez-Mosquera, M. I. and D. Hornero-Mendez (1994) Changes in carotenoid esterification during the fruit ripening of Capsicum annuum Cv. Bola. J. Agric. Food Chem. 42: 640–644.
Pérez-Gálvez, A. and M. I. Minguez-Mosquera (2005) Esterification of xanthophylls and its effect on chemical behavior and bioavailability of carotenoids in the human. Nutr. Res. 25: 631–640.
Subagio, A. and N. Morita (2003) Prooxidant activity of lutein and its dimyristate esters in corn triacylglyceride. Food Chem. 81: 97–102.
Fernández-García, E., M. I. Mínguez-Mosquera, and A. Pérez-Gálvez (2007) Changes in composition of the lipid matrix produce a differential incorporation of carotenoids in micelles. Interaction effect of cholesterol and oil. Innov Food Sci. Emerg. Technol. 8: 379–384.
Melgar-Lalanne, G., A. J. Hernández-Álvarez, M. Jiménez-Fernández, and E. Azuara (2017) Oleoresins from Capsicum spp.: extraction methods and bioactivity. Food Bioprocess Technol. 10: 51–76.
Arimboor, R., R. B. Natarajan, K. R. Menon, L. P. Chandrasekhar, and V. Moorkoth (2015) Red pepper (Capsicum annuum) carotenoids as a source of natural food colors: analysis and stability—a review. J. Food Sci. Technol. 52: 1258–1271.
Perva-Uzunalic, A., M. Skerget, B. Weinreich, and Z. Knez (2004) Extraction of chilli pepper (var. Byedige) with supercritical CO2: Effect of pressure and temperature on capsaicinoid and colour extraction efficiency. Food Chem. 87: 51–58.
Molina-Torres, J., A. García-Chávez, and E. Ramírez-Chávez (1999) Antimicrobial properties of alkamides present in flavouring plants traditionally used in Mesoamerica: affinin and capsaicin. J. Ethnopharmacol. 64: 241–248.
Sricharoen, P., N. Lamaiphan, P. Patthawaro, N. Limchoowong, S. Techawongstien, and S. Chanthai (2017) Phytochemicals in Capsicum oleoresin from different varieties of hot chilli peppers with their antidiabetic and antioxidant activities due to some phenolic compounds. Ultrason. Sonochem. 38: 629–639.
Bae, H., G. K. Jayaprakasha, K. Crosby, J. L. Jifon, and B. S. Patii (2012) Influence of extraction solvents on antioxidant activity and the content of bioactive compounds in non-pungent peppers. Plant Foods Hum. Nutr. 67: 120–128.
Özyildiz, F., S. Karagönlü, G. Basal, A. Uzel, and O. Bayraktar (2013) Micro-encapsulation of ozonated red pepper seed oil with antimicrobial activity and application to nonwoven fabric. Lett. Appl. Microbiol. 56: 168–179.
Madhumathy, A. P., A. A. Aivazi, and V. A. Vijayan (2007) Larvicidal efficacy of Capsicum annum against Anopheles stephensi and Culex quinquefasciatus. J. Vector Borne Dis. 44: 223–226.
Maliszewska, J. and E. Tęgowska (2012) Capsaicin as an organophosphate synergist against Colorado potato beetle (Leptinotarsa decemlineata Say). J. Plant Prot. Res. 52: 28–34.
Koleva-Gudeva, L., S. Mitrev, V. Maksimova, and D. Spasov (2013) Content of capsaicin extracted from hot pepper (Capsicum annuum ssp. microcarpum L.) and its use as an ecopesticide. Hem. Ind. 67: 671–675.
Fitzgerald, C. S., P. D. Curtis, M. E. Richmond, and J. A. Dunn (1995) Effectiveness of Capsaicin as a repellent to birdseed consumption by gray squirrels. National Wildlife Research Center Repellents Conference. August 8–10. Denver, Colorado, USA.
Andelt, W. F., K. P. Burnham, and D. L. Baker (1994) Effectiveness of capsaicin and bitrex repellents for deterring browsing by captive mule deer. J. Wildl. Manage. 58: 330–334.
Shumake, S. A., R. T. Sterner, and S. E. Gaddis (2000) Repellents to reduce cable gnawing by wild Norway rats. J. Wildl. Manage. 64: 1009–1013.
Kobata, K., K. Saito, H. Tate, A. Nashimoto, H. Okuda, I. Takemura, K. Miyakawa, M. Takahashi, K. Iwai, and T. Watanabe (2010) Long-chain N-vanillyl-acylamides from Capsicum oleoresin. J. Agric. Food Chem. 58: 3627–3631.
Chinn, M. S., R. R. Sharma-Shivappa, and J. L. Cotter (2011) Solvent extraction and quantification of capsaicinoids from Capsicum chinense. Food Bioprod. Process. 89: 340–345.
Rocha-Uribe, J. A., J. I. Novelo-Pérez, and C. A. Ruiz-Mercado (2014) Cost estimation for CO2 supercritical extraction systems and manufacturing cost for habanero chili. J. Supercrit. Fluids. 93: 38–41.
Acknowledgements
This work was supported by SEP-CONACYT grant 242544-2014. T.D-V. thanks CONACYT for her doctoral fellowship. The authors would like to express their gratitude to Dr. Torres and Mr. Rosales for their generous gifts of vanillylamine hydrochloride and Capsicum oleoresin.
The authors declare no conflict of interest.
Neither ethical approval nor informed consent was required for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Diaz-Vidal, T., Rosales-Rivera, L.C., Mateos-Díaz, J.C. et al. A Series of Novel Esters of Capsaicin Analogues Catalyzed by Candida antarctica Lipases. Biotechnol Bioproc E 25, 94–103 (2020). https://doi.org/10.1007/s12257-019-0290-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-019-0290-4