Abstract
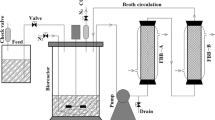
To promote its performance during acetone-butanol-ethanol (ABE) fermentation, Clostridium acetobutylicum (ATCC 824) was immobilized on a thin-shell silk cocoon (TSC). As a residual from the silk industry, TSC offers a cheap, biocompatible support material. The adsorbed C. acetobutylicum cells digested the TSCs into amino acids as a nitrogen source. It was shown that TSC might promote the phase shift to acetone in the ABE fermentation. At an initial reducing sugar concentration of 90 g/L, the ABE productivity of the immobilized cell culture on TSC (IC-TSC) in batch fermentation was 0.18 g/L/h, and the solvent mixture comprised 6.1 g/L acetone, 15.9 g/L butanol, and 1.9 g/L ethanol. Repeated 4-cycle batch fermentation using IC-TSC significantly improved the ABE productivity. After 48 h of cyclic fermentation, the maximum ABE productivity was 0.43 g/L/h with acetone, butanol and ethanol concentrations of 6.6, 12.9, and 1.1 g/L, respectively.
Similar content being viewed by others
References
Sukumaran, R. K., L. D. Gottumukkala, K. Rajasree, D. Alex, and A. Pandey (2011) Butanol fuel from biomass: Revisiting ABE fermentation. pp. 571–586. In: A. Pandey, C. Larroche, S. C Ricke, C.-G. Dussap, and E. Gnansounou (eds.). Biofuels Alternative Feedstocks and Conversion Processes. Academic Press, Elsevier, Amsterdam, Netherlands.
Qureshi, N., B. C. Saha, B. Dien, R. E. Hector, and M. A. Cotta (2010) Production of butanol (a biofuel) from agricultural residues: Part I–Use of barley straw hydrolysate. Biomass Bioenerg. 34: 559–565.
Szwaja, S. and J. D. Naber (2010) Combustion of n-butanol in a spark-ignition IC engine. Fuel 89: 1573–1582.
Sengupta, D. and R. W. Pike (2012) Chemicals from biomass, pp. 1043–1090. In: W.-Y. Chen, J. Seiner, T. Suzuki, and M. Lackner (eds.). Handbook of Climate Change Mitigation. Springer, New York, USA.
Jin, C., M. Yao, H. Liu, C.-F. F. Lee, and J. Ji (2011) Progress in the production and application of n-butanol as a biofuel. Renew. Sust. Energ. Rev. 15: 4080–4106.
Phisalaphong, M., R. Budiraharjo, P. Bangrak, J. Mongkolkajit, and S. Limtong (2007) Alginate-loofa as carrier matrix for ethanol production. J. Biosci. Bioeng. 104: 214–217.
Zhu, Y. (2007) Immobilized cell fermentation for production of chemicals and fuels. pp. 373–396. In: Y. Shang-Tian (ed.). Bioprocessing for Value-Added Products from Renewable Resources, Elsevier, Amsterdam, Netherlands.
Kourkoutas, Y., A. Bekatorou, I. M. Banat, R. Marchant, and A. A. Koutinas (2004) Immobilization technologies and support materials suitable in alcohol beverages production: A review. Food Microbiol. 21: 377–397.
He, Q., and H. Chen (2013) Improved efficiency of butanol production by absorbed lignocellulose fermentation. J. Biosci. Bioeng. 115: 298–302.
Rattanapan, A., S. Limtong, and M. Phisalaphong (2011) Ethanol production by repeated batch and continuous fermentations of blackstrap molasses using immobilized yeast cells on thin-shell silk cocoons. Appl. Energy 88: 4400–4404.
Tsujimoto, K., H. Takagi, M. Takahashi, H. Yamada, and S. Nakamori (2001) Cryoprotective effect of the serine-rich repetitive sequence in silk protein sericin. J. Biochem. 129: 979–986.
Aramwit, P., S. Kanokpanont, W. de-Eknamkul, K. Kamei, and T. Srichana (2009) The effect of sericin with variable amino-acid content from different silk strains on the production of collagen and nitric oxide. J. Biomater. Sci. Polym. Ed. 20: 1295–1306.
Sutcharit, A., C. Satirapipathkul, C. Muangnapoh, V. Burapatana, and M. Phisalaphong (2010) Butanol production from sugarcane juice by Clostridium spp. in batch fermentation. KKU Eng. J. 37: 339–347.
Shaheen, R., M. Shirley, and D. T. Jones (2000) Comparative fermentation studies of industrial strains belonging to four species of solvent-producing clostridia. J. Mol. Microbiol. Biotechnol. 2: 115–124.
Yen, H. -W., R. -J. Li, and T. -W. Ma (2011) The development process for a continuous acetone–butanol–ethanol (ABE) fermentation by immobilized Clostridium acetobutylicum. J. Taiwan Inst. Chem. Eng. 42: 902–907.
Huesemann, M. H., L. -J. Kuo, L. Urquhart, G. A. Gill, and G. Roesijadi (2004) Acetone-butanol fermentation of marine macroalgae. Bioresour. Technol. 108: 305–309.
Ezeji, T. C., N. Qureshi, and H. P. Blaschek (2004) Butanol fermentation research: upstream and downstream manipulations. Chem. Rec. 4: 305–314.
Yen, H. -W. and R. -J. Li (2011) The effects of dilution rate and glucose concentration on continuous acetone–butanol–ethanol fermentation by Clostridium acetobutylicum immobilized on bricks. J. Chem. Technol. Biotechnol. 86: 1399–1404.
Li, L., H. Ai, S. Zhang, S. Li, Z. Liang, Z. -Q. Wu, S. -T. Yang, and J. -F. Wang (2013) Enhanced butanol production by coculture of Clostridium beijerinckii and Clostridium tyrobutyricum. Bioresour. Technol. 143: 397–404.
Welsh, F. W., R. E. Williams, and I. A. Veliky (1987) Solid carriers for a Clostridium acetobutylicum that produces acetone and butanol. Enz. Microb. Technol. 9: 500–502.
Wagner, A. O., P. Hohlbrugger, P. Lins, and P. Illmer (2012) Effects of different nitrogen sources on the biogas production–a lab-scale investigation. Microbiol. Res. 167: 630–636.
Barker, H. A. (1981) Amino acid degradation by anaerobic bacteria. Annu. Rev. Biochem. 50: 23–40.
Teshome, A., F. Vollrath, S. K. Raina, J. M. Kabaru, and J. Onyari (2012) Study on the microstructure of African wild silk cocoon shells and fibers. Int. J. Biol. Macromol. 50: 63–68.
Zhang, Y. -Q. (2002) Applications of natural silk protein sericin in biomaterials. Biotechnol. Adv. 20: 91–100.
Chen, Y., T. Zhou, D. Liu, A. Li, S. Xu, Q. Liu, B. Li, and H. Ying (2013) Production of butanol from glucose and xylose with immobilized cells of Clostridium acetobutylicum. Biotechnol. Bioproc. Eng. 18: 234–241.
Maddox, I. S. (1989) The Acetone-butanol-ethanol fermentation: Recent progress in technology. Biotechnol. Genet. Eng. Rev. 7: 189–220.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kittithanesuan, N., Phisalaphong, M. Enhanced acetone-butanol production from sugarcane juice by immobilized Clostridium acetobutylicum (ATCC 824) on thin-shell silk cocoons. Biotechnol Bioproc E 20, 599–607 (2015). https://doi.org/10.1007/s12257-014-0709-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-014-0709-x