Abstract
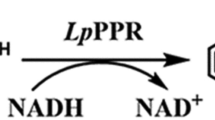
Lysine decarboxylase (LDC) exhibits a significant role in cadaverine (1,5-pentanediamine, diaminopentane) production from lysine. In this study, an error-prone PCR and DNA shuffling were performed to improve the activity of LDC from Hafnia alvei AS1.1009 for cadaverine production. A sensitive high-throughput screening strategy based on a pH indicator was established for directed evolution of LDC. Several improved mutants were obtained from directed evolution and LDCV147F/E583G mutant showed highest activity to catalyze lysine to cadaverine. This mutant showed 1.62-fold high LDC activity when compared to wild-type. Further analysis by site-directed mutagenesis reveled that only the mutant E583G was sufficient for higher catalytic activity. Wild type LDC and mutant LDCE583G were purified by an improved method including hydrophobic chromatography. These purified enzymes were characterized and the kinetic parameters were compared between LDCE583G and WT LDC. V max of LDCE583G was 1.32-fold higher than that of WT LDC. Use of LDCE583G mutant showed 1.48-fold improved productivity of cadaverine when compared to wild type. The concentration of cadaverine in E. coli JM109/pTrc99a-ldc2-41 was 63.9 g/L with conversion yield of 93.4% during 5 h. These results indicate that the mutation has positive effects on improving LDC activity and a potential candidate for cadaverine production.
Similar content being viewed by others
References
Flores, F. J., J. Rincon, and J. F. Martin (2003) Characterization of the iron-regulated desA promoter of Streptomyces pilosus as a system for controlled gene expression in actinomycetes. Microb. Cell. Fact. 2: 5.
Kind, S., S. Kreye, and C. Wittmann (2011) Metabolic engineering of cellular transport for overproduction of the platform chemical 1,5-diaminopentane in Corynebacterium glutamicum. Metab. Eng. 13: 617–627.
Mimitsuka, T., H. Sawai, M. Hatsu, and K. Yamada (2007) Metabolic engineering of Corynebacterium glutamicum for cadaverine fermentation. Biosci. Biotech. Bioch. 71: 2130–2135.
Tateno, T., Y. Okada, T. Tsuchidate, T. Tanaka, H. Fukuda, and A. Kondo(2009) Direct production of cadaverine from soluble starch using Corynebacterium glutamicum coexpressing amylase and lysine decarboxylase. Appl. Microbiol. Biotechnol. 82: 115–122.
Kind, S., W. K. Jeong, H. Schroder, O. Zelder, and C. Wittmann (2010) Identification and elimination of the competing N-Acetyldiaminopentane Pathway for improved production of diaminopentane by Corynebacterium glutamicum. Appl. Environ. Microb. 76: 5175–5180.
Buschke, N., H. Schroeder, and C. Wittmann (2011) Metabolic engineering of Corynebacterium glutamicum for production of 1,5-diaminopentane from hemicellulose. Biotechnol. J. 6: 306–317.
Cadwell, R. C. and G. F. Joyce (1994) Mutagenic PCR. PCR Methods. Appl. 3: S136-S140.
Stemmer, W. P. (1994) Rapid evolution of a protein in vitro by DNA shuffling. Nature 370: 389–391.
Wang, C., H. L. Zhang, H. Cai, Z. H. Zhou, Y. L. Chen, Y. L. Chen, and P. K. Ouyang (2014) Succinic acid production from corn cob hydrolysates by genetically engineered Corynebacterium glutamicum. Appl. Biochem. Biotechnol. 172: 340–343.
Stemmer, W. P. (1994) DNA shuffling by random fragmentation and reassembly: In vitro recombination for molecular evolution. PNAS 91: 10747–10751.
Yumiko, T., O. Motoko, S. Takeyoshi, M. Koji, T. Toshio, and K. Yoshiyuki (1999) Novel characteristics of Selenomonas ruminantium lysine decarboxylase capable of decarboxylating both L-Lysine and L-Ornithine. Biosci. Biotechnol. Biochem. 63: 1063–1069.
Phan, A. P., T. T. Ngo, and H. M. Lenhoff (1982) Spectrophotometric assay for lysine decarboxylase. Anal. Biochem. 120: 193–197.
Bradford, M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
Krömer, J. O., M. Fritz, E. Heinzle, and C. Wittmann (2005) In vivo quantification of intracellular amino acids and intermediates of the methionine pathway in Corynebacterium glutamicum. Anal. Biochem. 340: 171–173.
Önal, A. (2007) A review: Current analytical methods for the determination of biogenic amines in foods. Food Chem. 103: 1475–1486.
Yildirim, H. K., A. Üren, and U. Yücel (2007) Evaluation of biogenic amines in organic and non-organic wines by HPLC OPA derivatization. Food Technol. Biotechnol. 45: 62–68.
Qian, Z. G., X. X. Xia, and S. Y. Lee (2009) Metabolic engineering of Escherichia coli for the production of putrescine: A four carbon diamine. Biotechnol. Bioeng. 104: 651–662.
Powell, K. A., W. P. Sandra, S. B. Del Cardayre, W. P. Stemmer, M. B. Tobin, P. F. Longchamp, and G. W. Huisman (2001) Directed evolution and biocatalysis. Angewandte Chemie International Edition, 40: 3948–3959.
Zhao, H. M., K. Chockalingam, and Z. Chen (2002) Directed evolution of enzymes and pathways for industrial biocatalysis. Curr. Opin. Biotech. 13: 104–110.
Jaeger, K.E. and T. Eggert (2004) Enantioselective biocatalysis optimized by directed evolution. Curr. Opin. Biotech. 15: 305–315.
An, Y.F., J. F. Ji, W. F. Wu, R. B. Huang, Y. T. Wei, and Z. L. Xiu (2008) Random mutagenesis and recombination of sam1 gene by integrating error-prone PCR with staggered extension process. Biotechnol. Lett. 30: 1227–1232.
Author information
Authors and Affiliations
Corresponding author
Additional information
These author’s contributed equally to the present work.
Rights and permissions
About this article
Cite this article
Wang, C., Zhang, K., Zhongjun, C. et al. Directed evolution and mutagenesis of lysine decarboxylase from Hafnia alvei AS1.1009 to improve its activity toward efficient cadaverine production. Biotechnol Bioproc E 20, 439–446 (2015). https://doi.org/10.1007/s12257-014-0690-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-014-0690-4