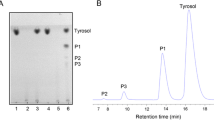
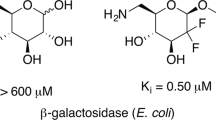
Abstract
Acyclic nucleoside analogs constitute an important group of antiviral agents. However, these nucleoside drugs suffer from poor water solubility and low oral bioavailability in the clinic use. In the present work, the enzymatic synthesis of the water-soluble galactosidic prodrugs of acyclic nucleosides by using bovine liver β-galactosidase was described. In the enzymatic transgalactosylation between acyclovir (ACV) and o-nitrophenyl β-galactopyranoside (oNPGal), the optimum enzyme dosage, buffer pH, temperature and molar ratio of ACV to oNPGal were 0.225 U/mL, 7.0, 40°C and 2.5, respectively, under which the initial reaction rate and the yield reached 0.40 mM/h and 29%, respectively. In addition, this enzyme could accept ganciclovir (GCV) and penciclovir (PCV) as substrates, affording the corresponding 4’-β-galactosylated derivatives with the yields of 26% and 71%, respectively.
Similar content being viewed by others
References
Freeman, S. and J. M. Gardiner (1996) Acyclic nucleosides as antiviral compounds. Mol. Biotechnol. 5: 125–137.
De Clercq, E. (2004) Antiviral drugs in current clinical use. J. Clin. Virol. 30: 115–133.
De Clercq, E. and H. J. Field (2006) Antiviral prodrugs — the development of successful prodrug strategies for antiviral chemotherapy. Br. J. Pharmacol. 147: 1–11.
Rautio, J., H. Kumpulainen, T. Heimbach, R. Oliyai, D. Oh, T. Jarvinen, and J. Savolainen (2008) Prodrugs: Design and clinical applications. Nat. Rev. Drug Discovery 7: 255–270.
Santos, C. R., R. Capela, C. S. G. P. Pereira, E. Valente, B. Luis Gouveia, C. Pannecouque, E. D. Clercq, R. Moreira, and P. Gomes (2009) Structure-activity relationships for dipeptide prodrugs of acyclovir: Implications for prodrug design. Eur. J. Med. Chem. 44: 2339–2346.
Colla, L., E. D. Clercq, R. Busson, and H. Vanderhaeghe (1983) Synthesis and antiviral activity of water-soluble esters of acyclovir [9-[(2-hydroxyethoxy )methyl]guanine]. J. Med. Chem. 26: 602–604.
Patel, K., S. Trivedi, S. Luo, Xiaodong Zhu, D. Pal, E. R. Kern, and A. K. Mitra (2005) Synthesis, physicochemical properties and antiviral activities of ester prodrugs of ganciclovir. Int. J. Pharm. 305: 75–89.
Curran, M. and S. Noble (2001) Valganciclovir. Drugs. 61: 1145–1150.
Wu, C. Z., J. H. Jang, M. Woo, J. S. Ahn, J. S. Kim, and Y. S. Hong (2012) Enzymatic glycosylation of non-benzoquinone geldanamycin analogs via Bacillus UDP-glycosyltransferase. Appl. Environ. Microbiol. 78: 7680–7686.
Abraham, R., N. Aman, R. von Borstel, M. Darsley, B. Kamireddy, J. Kenten, G. Morris, and R. Titmas (1994) Conjugates of COL-1 monoclonal antibody and β-D-galactosidase can specifically kill tumor cells by generation of 5-fluorouridine from the prodrug β-D-galactosyl-5-fluorouridine. Cell Biochem. Biophys. 24: 127–133.
Watanabe, K. A., A. Matsuda, M. J. Halat, D. H. Hollenberg, J. S. Nisselbaum, and J. J. Fox (1981) Nucleosides. 114. 5′-O-Glucuronides of 5-fluorouridine and 5-fluorocytidine. Masked precursors of anticancer nucleosides. J. Med. Chem. 24: 893–897.
Li, N., T. J. Smith, and M. H. Zong (2010) Biocatalytic transformation of nucleoside derivatives. Biotechnol. Adv. 28: 348–366.
Binder, W. H., H. Kahlig, and W. Schmid (1995) Galactosylation by use of β-galactosidase: Enzymatic syntheses of disaccharide nucleosides. Tetrahedron: Asymm. 6: 1703–1710.
Andreotti, G., A. Trincone, and A. Giordano (2007) Convenient synthesis of β-galactosyl nucleosides using the marine β-galactosidase from Aplysia fasciata. J. Mol. Catal. B: Enzym. 47: 28–32.
Blazek, J., P. Jansa, O. Baszczynski, M. M. Kaiser, M. Otmar, M. Krecmerova, M. Drancinsky, A. Holy, and B. Kralova (2012) An enzymatic glycosylation of nucleoside analogues using β-galactosidase from Escherichia coli. Bioorg. Med. Chem. 20: 3111–3118.
Ye, M., L. Q. Yan, N. Li, and M. H. Zong (2012) Facile and regioselective enzymatic 5′-galactosylation of pyrimidine 2′-deoxy-nucleosides catalyzed by β-glycosidase from bovine liver. J. Mol. Catal. B: Enzym. 79: 35–40.
Yan, L. Q., N. Li, and M. H. Zong (2012) First and facile enzymatic synthesis of β-fucosyl-containing disaccharide nucleosides through β-galactosidase-catalyzed regioselective glycosylation. J. Biotechnol. 164: 371–375.
Ye, M., C. Y. Yu, N. Li, and M. H. Zong (2011) Highly regioselective glucosylation of 2′-deoxynucleosides by using the crude β-glycosidase from bovine liver. J. Biotechnol. 155: 203–208.
National Institute of Advanced Industrial Science and Technology (AIST), Japan. Spectral database for organic compounds, SDBS. http://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_index.cgi?lang=eng.
Lichtenthaler, F. W., W. Eberhard, and S. Braun (1981) Nucleosides, 45.-Assignment of glycosylation sites in O-hexopyranosylribonucleosides by 13C NMR. Tetrahedron Lett. 22: 4401–4404.
Chen, L., N. Li, and M. H. Zong (2012) A Glucose-tolerant β-glucosidase from Prunus domestica seeds: purification and characterization. Proc. Biochem. 47: 127–132.
Zeng, Q. M., N. Li, and M. H. Zong (2010) Highly regioselective galactosylation of floxuridine catalyzed by β-galactosidase from bovine liver. Biotechnol. Lett. 32: 1251–1254.
Isobe, K., M. Yamashita, S. Chiba, N. Takahashi, and T. Koyama (2013) Characterization of new β-galactosidase from acidophilic fungus, Teratosphaeria acidotherma AIU BGA-1. J. Biosci. Bioeng. 116: 293–297.
van Rantwijk, F., M. W. V. Oosterom, and R. A. Sheldon (1999) Glycosidase-catalysed synthesis of alkyl glycosides. J. Mol. Catal. B: Enzym. 6: 511–532.
Kazlauskas, R. J. (2000) Molecular modeling and biocatalysis: Explanations, predictions, limitations, and opportunities. Curr. Opin. Chem. Biol. 4: 81–88.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yan, LQ., Li, N. & Zong, MH. First enzymatic galactosylation of acyclic nucleoside drugs by β-galactosidase: Synthesis of water-soluble β-D-galactosidic prodrugs. Biotechnol Bioproc E 19, 586–591 (2014). https://doi.org/10.1007/s12257-013-0823-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-013-0823-1