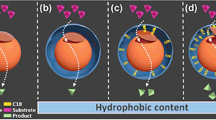
Abstract
Candida rugosa lipase (CRL) is one of the most widely used lipases. To enhance the catalytic abilities of CRL in both aqueous and non-aqueous phases, hollow silica microspheres (HSMSs) with a pore size of 18.07 nm were used as an immobilization support, and aldehydecontaining dextrans were employed to further cross-link the adsorbed CRL. In the experimental ranges examined, the loading amount of lipase linearly increased to 171 ± 3.4 mgprotein/gsupport with the CRL concentration and all the adsorption equilibriums were reached within 30 min. After simple cross-linking, the tolerance to pH 4.0 ∼ 8.0 as well as the thermal stability of immobilized CRL at 40 ∼ 80°C were both substantially increased, and 82 ± 2.1% activity remaining after the sixth reuse. The immobilized CRL was successfully applied to the resolution of racemic ibuprofen in non-aqueous phase. The initial reaction rate increased by 1.4- and 3.6-fold compared with the rates of adsorbed and native lipases, respectively. Furthermore, the R-ibuprofen was obtained at ee > 93%, and the enantiomeric ratio reached E > 140 at the conversion of 50 ± 1.5% within 48 h.
Similar content being viewed by others
References
Jin, D., K. Park, J. H. Lee, K. Song, J. Kim, M. L. Seo, and J. H. Jung (2011) The selective immobilization of curcumin onto the internal surface of mesoporous hollow silica particles by covalent bonding and its controlled release. J. Mater. Chem. 21: 3641–3645.
Murty, V. R., J. Bhat, and P. K. A. Muniswaran (2002) Hydrolysis of oils by using immobilized lipase enzyme: A review. Biotechnol. Bioproc. Eng. 7: 57–66.
Salis, A., M. F. Casula, M. S. Bhattacharyya, M. Pinna, V. Solinas, and M. Monduzzi (2010) Physical and chemical lipase adsorption on SBA-15: Effect of different Interactions on enzyme loading and catalytic performance. ChemCatChem. 2: 322–329.
Zhou, C., S. Zhu, X. Wu, B. Jiang, T. Cen, and S. Shen (2010) Post-immobilization of modified macromolecular reagents using assembled penicillin acylase for microenvironmental regulation of nanopores and enhancement of enzyme stability. Biotechnol. Bioproc. Eng. 15: 376–382.
Cao, Z., J. Zhang, J. Zeng, L. Sun, F. Xu, Z. Cao, L. Zhang, and D. Yang (2009) Mesoporous silica hollow sphere (MSHS) for the bioelectrochemistry of horseradish peroxidase. Talanta 77: 943–947.
Suarez, F. J., M. Sevilla, S. Alvarez, T. Valdes-Solis, and A. B. Fuertes (2007) Synthesis of highly uniform mesoporous submicrometric capsules of silicon oxycarbide and silica. Chem. Mater. 19: 3096–3098.
Zoldesi, C. I. and A. Imhof (2005) Synthesis of monodisperse colloidal spheres, capsules, and microballoons by emulsion templating. Adv. Mater. 17: 924–928.
Yu, J., L. Ge, P. Dai, S. Ge, and S. Liu (2010) A novel enzyme biosensor for glucose based on rhodanine derivative chemiluminescence system and mesoporous hollow silica microspheres receptor. Biosens. Bioelectron. 25: 2065–2070.
Ni, D., L. Wang, Y. Sun, Z. Guan, S. Yang, and K. Zhou (2010) Amphiphilic hollow carbonaceous microspheres with permeable shells. Angew. Chem. Int. Ed. 49: 4223–4227.
Jin, C., Y. Guo, S. Zhang, and W. Shen (2012) Synthesis of large spherical mesoporous silica using tween-80 and starch hydrolysis solution. Adv. Sci. Lett. 5: 204–207.
Lee, D. H., J. M. Kim, H. Y. Shin, S. W. Kang, and S. W. Kim (2006) Biodiesel production using a mixture of immobilized Rhizopus oryzae and Candida rugosa lipases. Biotechnol. Bioproc. Eng. 11: 522–525.
Benjamin, S. and A. Pandey (1998) Candida rugosa lipases: Molecular biology and versatility in biotechnology. Yeast 14: 1069–1087.
Lu, S., J. He, and X. Guo (2010) Architecture and performance of mesoporous silica-lipase hybrids via non-covalent interfacial adsorption. AIChE J. 56: 506–514.
Salis, A., D. Meloni, S. Ligas, M. F. Casula, M. Monduzzi, V. Solinas, and E. Dumitriu (2005) Physical and chemical adsorption of Mucor javanicus lipase on SBA-15 mesoporous silica. Synthesis, structural characterization and activity performance. Langmuir 21: 5511–5516.
He, J., Y. Xu, H. Ma, Q. Zhang, D. G. Evans, and X. Duan (2006) Effect of surface hydrophobicity/hydrophilicity of mesoporous supports on the activity of immobilized lipase. J. Colloid Int. Sci. 298: 780–786.
Serra, E., A. Mayoral, Y. Sakamoto, R. M. Blanco, and I. Diaz (2008) Immobilization of lipase in ordered mesoporous materials: Effect of textural and structural parameters. Microp. Mesop. Mater. 114: 201–213.
Yiu, H. and P. A. Wright (2005) Enzymes supported on ordered mesoporous solids: A special case of an inorganic-organic hybrid. J. Mater. Chem. 15: 3690–3700.
Gao, S. L., Y. J. Wang, X. Diao, G. S. Luo, and Y. Y. Dai (2010) Effect of pore diameter and cross-linking method on the immobilization efficiency of Candida rugosa lipase in SBA-15. Bioresour. Tech. 101: 3830–3837.
Migneault, I., C. Dartiguenave, M. J. Bertrand, and K. C. Waldron (2004) Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. BioTechniques 37:790–802.
Kim, M. I., J. Kim, J. Lee, H. Jia, H. Bin Na, J. K. Youn, J. H. Kwak, A. Dohnalkova, J. W. Grate, P. Wang, T. Hyeon, H. G. Park, and H. N. Chang (2007) Crosslinked enzyme aggregates in hierarchically-ordered mesoporous silica: A simple and effective method for enzyme stabilization. Biotechnol. Bioeng. 96: 210–218.
Rodrigues, D. S., A. A. Mendes, W. S. Adriano, L. Goncalves, and R. Giordano (2008) Multipoint covalent immobilization of microbial lipase on chitosan and agarose activated by different methods. J. Mol. Catal. B: Enzym. 51: 100–109.
de la Casa, R. M., J. M. Guisan, J. M. Sanchez-Montero, and J. V. Sinisterra (2002) Modification of the activities of two different lipases from Candida rugosa with dextrans. Enz. Microb. Technol. 30: 30–40.
de Maria, P. D., J. V. Sinisterra, J. M. Sanchez-Montero, M. Lotti, F. Valero, and A. R. Alcantara (2006) Acyl transfer strategy for the biocatalytical characterisation of Candida rugosa lipases in organic solvents. Enz. Microb. Technol. 38: 199–208.
Merabet-Khelassi, M., Z. Houiene, L. Aribi-Zouioueche, and O. Riant (2012) Green methodology for enzymatic hydrolysis of acetates in non-aqueous media via carbonate salts. Tetrahedron: Asymmetry 23: 828–833.
Gawronski, J., K. Gawronska, P. Skowronek, U. Rychlewska, B. Warzajtis, J. Rychlewski, M. Hoffmann, and A. Szarecka (1997) Factors affecting conformation of (R,R)-tartaric acid ester, amide and nitrile derivatives. X-ray diffraction, circular dichroism, nuclear magnetic resonance and ab initio studies. Tetrahedron 53: 6113–6144.
Basri, M., K. Ampon, W. Yunus, C. Razak, and A. B. Salleh (1994) Immobilization of hydrophobic lipase derivatives on to organic polymer beads. J. Chem. Technol. Biotechnol. 59: 37–44.
Germain, P., T. Slagmolen, and R. R. Crichton (1989) Relation between stabilization and rigidification of the 3-dimensional structure of an enzyme. Biotechnol. Bioeng. 33: 563–569.
Kobayashi, M. and K. Takatsu (1994) Cross-linked stabilization of trypsin with dextran-dialdehyde. Biosci. Biotechnol. Biochem. 58: 275–278.
Zhao, H. and N. D. Heindel (1991) Determination of degree of substitution of formyl groups in polyaldehyde dextran by the hydroxylamine hydrochloride method. Pharm. Res. 8: 400–402.
Bradford, M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
Ozyilmaz, G. (2009) The effect of spacer arm on hydrolytic and synthetic activity of Candida rugosa lipase immobilized on silica gel. J. Mol. Catal. B: Enzym. 56: 231–236.
Lopez N, M. A. Pernas, L. M. Pastrana, A. Sanchez, F. Valero, and M. L. Rua (2004) Reactivity of pure Candida rugosa lipase isoenzymes (Lip1, Lip2, and Lip3) in aqueous and organic media. Influence of the isoenzymatic profile on the lipase performance in organic media. Biotechnol. Progr. 20: 65–73.
Natalello, A., D. Ami, S. Brocca, M. Lotti, and S. M. Doglia (2005) Secondary structure, conformational stability and glycosylation of a recombinant Candida rugosa lipase studied by fouriertransform infrared spectroscopy. Biochem. J. 385: 511–517.
Yu, H. W., J. C. Wu, and C. B. Ching (2004) Enhanced activity and enantioselectivity of Candida rugosa lipase immobilized on macroporous adsorptive resins for ibuprofen resolution. Biotechnol. Lett. 26: 629–633.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ren, L., Jia, H., Yu, M. et al. Enhanced catalytic ability of Candida rugosa lipase immobilized on pore-enlarged hollow silica microspheres and cross-linked by modified dextran in both aqueous and non-aqueous phases. Biotechnol Bioproc E 18, 888–896 (2013). https://doi.org/10.1007/s12257-013-0044-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-013-0044-7