Abstract
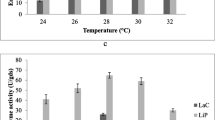
Lignin and manganese peroxidase (LiP, MnP) and laccase production by Phanerocheate chrysosporium was optimized by response surface methodology for brewery waste and apple pomace. The effect of moisture, copper sulphate, and veratryl alcohol (VA) concentrations on enzyme production was studied. Moisture and VA had significant positive effect on MnP and LiP production and the viability of P. chrysosporium (p < 0.05) and copper sulphate produced a negative effect. However, moisture and copper sulphate had a significant positive (p < 0.05) effect on laccase production, but VA had an insignificant positive effect (p < 0.05). Higher values of MnP, LiP and viability of P. chrysosporium on apple pomace (1287.5 U MnP/gds (units/gram dry substrate), 305 U LiP/gds, and 10.38 Log 10 viability) and brewery waste (792 U MnP/gds and 9.83 Log 10 viability) were obtained with 80% moisture, 3 mmol/kg VA, and 0.5 mmol/kg copper. LiP production in brewery waste (7.87 U/gds) was maximal at 70% moisture, 2 mmol/kg VA, and 1 mmol/kg copper. Higher production of laccase in apple pomace (789 U/gds) and brewery waste (841 U/gds) were obtained with 80% moisture, 3 mmol/kg VA, and 1.5 mmol/kg copper. Thus, moisture along with VA and copper sulphate was pertinent for the production of ligninolytic enzymes and increased cell viability.
Similar content being viewed by others
References
Rodrìguez Couto, S. and J. L. Toca Herrera (2006) Industrial and biotechnological applications of laccases: A review. Biotechnol. Adv. 24: 500–513.
Casa, R., A. D’Annibale, F. Pieruccetti, S. R. Stazi, G. Giovannozzi Sermanni, and B. Lo Cascio (2003) Reduction of the phenolic components in olive-mill wastewater by an enzymatic treatment and its impact on durum wheat (Triticum durum DESF.) germinability. Chemosphere 50: 959–966.
Barclay, C. D., R. L. Legge, and G. F. Farquhar (1993) Modelling the growth kinetics of Phanerochaete chrysosporium in submerged static culture. Appl. Environ. Microbiol. 59: 1887–1892.
Moredo, N., M. Lorenzo, A. Domýnguez, D. Moldes, C. Cameselle, and A. Sanroman (2003) Enhanced ligninolytic enzyme production and degrading capability of Phanerochaete chrysosporium and Trametes versicolor. World J. Microbiol. Biotechnol. 19: 665–669.
Hatakka, A. (1994) Lignin modifying enzymes from selected white-rot fungi: Production and role in lignin degradation. FEMS Microbiol. Rev. 13: 125–135.
Hacking, A. J. (1987) Economic Aspects of Biotechnology. Cambridge University Press, Cambridge.
Vendruscolo, F., P. C. M. Albuquerque, F. Streit, E. Esposito, and J. L. Ninow (2008) Apple pomace: A versatile substrate for biotechnological applications. Crit. Rev. Biotechnol. 28: 1–12.
Mussatto, S. I. (2009) Biotechnological potential of brewing industry by-products. pp. 313–326. In: P. S. Nigam and A. Pandey (eds.). Biotechnology for Agro-Industrial Residues Utilisation. Springer, Netherlands.
Viniegra-González, G., N. Favela-Torres, C. N. Aguilar, S. J. Romero-Gómez, G. Díaz-Godínez, and C. Augur (2003) Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochem. Eng. J. 13: 157–167.
Lundquist, K. and T. K. Kirk (1978) De novo synthesis and decomposition of veratryl alcohol by a lignin-degrading basidiomycete. Phytochem. 17: 1676.
Arora, D. S. and P. K. Gill (2001) Comparison of two assay procedures for lignin peroxides. Enz. Microb. Tech. 28: 602–605.
Alvarez, J. M., P. Canessa, R. A. Mancilla, R. Polanco, P. A. Santibánez, and R. Vicuna (2009) Expression of genes encoding laccase and manganese-dependent peroxidise in the fungus Ceriporiopsis subvermispora is mediated by an ACE1-like copperfist transcription factor. Fungal Genet. Biol. 46: 104–111.
Pandey, A. (2003) Solid state fermentation. Biochem. Eng. J. 13: 81–84.
Boza, A., Y. De la Cruz, G. Jordan, U. Jauregui-Haza, A. Aleman, and I. Caraballo (2000) Statistical optimization of a sustainedrelease matrix tablet of lobenzarit disodium. Drug. Dev. Ind. Pharm. 26: 1303–1307.
Singh, S. K., J. Dodge, M. J. Durrani, and M. A. Khan (1995) Optimization and characterization of controlled release pellets coated with experimental latex: I. Anionic drug. Int. J. Pharm. 125: 243–255.
Sanchez-Lafuente, C., S. Furlanetto, and M. Fernandez-Arevalo (2002) Didanosine extended-release matrix tablets: Optimization of formulation variables using statistical experimental design. Int. J. Pharm. 237: 107–118.
Francis, F., A. Sabu, K. M. Nampoothiri, S. Ramachandran, S. Ghosh, and G. Szakacs (2003) Use of response surface methodology for optimizing process parameters for the production of α-amylase by Aspergillus oryzae. Biochem. Eng. J. 107–115.
Gassara, F., S. K. Brar, R. D. Tyagi, M. Verma, and R. Y. Surampalli (2010) Screening of agro-industrial wastes to produce ligninolytic enzymes by Phanerocheate chrysosporium. Biochem. Eng. J. 49: 388–394.
Tien, M. and T. K. Kirk (1984) Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H2O2-requiring oxygenase. Proc. Natl. Acad. Sci. U S A. 81: 2280–2284.
Collins, P. J. and A. D. W. Dobson (1997) Regulation of laccase gene transcription in Trametes versicolor. Appl. Environ. Microbiol. 63: 3444–3450.
Fujian, X., C. H. Zhang, and L. Zuohn (2001) Solid-state production of lignin peroxidase (LiP) and manganese peroxidase (MnP) by Phanerochaete chrysosporium using steam exploded straw as substrate. Biores. Technol. 80: 149–151.
Thomas, H. A. (1942) Bacterial densities from fermentation tube tests. J. Am. Water Work Assoc. 34: 572–576.
Gill, P. K. and D. S. Arora (2003) Effect of culture conditions on manganese peroxidase production and activity by some white-rot fungi. J. Ind. Microbiol. Biotechnol. 30: 28–33.
Baldrian, P. (2003) Interactions of heavy metals with white-rot fungi. Enz. Microb. Technol. 32: 78–91.
Dittmer, J. K., N. J. Patel, S. W. Dhawale, and S. S. Dhawale (1997) Production of multiple laccase isoforms by Phanerochaete chrysosporium grown under nutrient sufficiency. FEMS. Microbiol. Lett. 149: 65–70.
De Jong, E., J. Field, and J. de Bont (1994) Aryl alcohols in the physiology of ligninolytic fungi. FEMS Microbiol. Rev. 13: 153–188.
Vasconcelos, A. F. D., A. M. Barbosa, R. F. H. Dekker, I. S. Scarminio, and M. I. Rezende (2000) Optimisation of laccase production by Botryosphaeria sp. in the presence of veratryl alcohol by the response-surface method. Proc. Biochem. 35: 1131–1138.
Galhaup, C., H. Wagner, B. Hinterstoisser, and D. Haltrich (2002) Increased production of laccase by the wood-degrading basidiomycete Trametes pubescens. Enz. Microb. Technol. 30: 529–536.
Tavares, A. P. M., M. A. Z. Coelho, J. A. P. Coutinho, and A. M. R. B. Xavier (2005) Laccase improvement in submerged cultivation: Induced production and kinetic modeling. J. Chem. Technol. Biotechnol. 80: 669–676.
Klonowska, A., J. Le Petit, and T. Tron (2001) Enhancement of minor laccases production in the basidiomycete Marasmius quercophilus C30. FEMS. Microbiol. Lett. 200: 25–30.
Verma, P. and D. Madamwar (2002) Production of lignolytic enzymes for dye decolourization by cocultivation of White rot fungi Pleurotus ostreatus and Phanerochaete chrysosporium under solid state fermentation. Appl. Biochem. Biotechnol. 102: 109–118.
Mai, C., U. Kües, and H. Militz (2004) Biotechnology in the wood industry. Appl. Microbiol. Biotechnol. 63: 477–494.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gassara, F., Brar, S.K., Tyagi, R.D. et al. Parameter optimization for production of ligninolytic enzymes using agro-industrial wastes by response surface method. Biotechnol Bioproc E 16, 343–351 (2011). https://doi.org/10.1007/s12257-010-0264-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-010-0264-z