Abstract
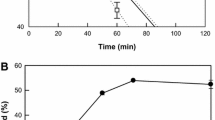
The effects of cell wall mutation on the oxygenation of linoleic acid (M.W. 280) by recombinant Escherichia coli expressing the CYP102A2 gene encoding self-sufficient P450 monooxygenase of Bacillus subtilis was investigated. After the CYP102A2 gene was heterologously expressed in E. coli W3110 and its isogenic lipopolysaccharide (LPS) structural mutant strains, their whole-cell biotransformation activities were compared. The mutants used in this study had previously been designated as MLK53, MLK1067, and MLK986. These strains carry one or two defined mutations in the secondary acyl fatty acids of the LPS lipid A constituent. The CYP102A2 gene was overexpressed in both wild type E. coli W3110 and its mutant strains, with the specific activity ranging from 1.7 to 2.1 U/mg protein. Interestingly, the whole-cell biotransformation activity of those recombinant biocatalysts differed significantly. Indeed, MLK986 possessing the tetraacylated LPS showed a higher oxygenation activity of linoleic acid than those in wild type or other mutant strains having hexa- or penta-acylated LPSs. These results suggest that the biotransformation efficiency of E. coli-based biocatalysts, especially for medium- to large-sized lipophilic organic substrates, can be enhanced via engineering their LPS, which is known to function as a formidable barrier for hydrophobic molecules.
Similar content being viewed by others
References
Blank, L. M., B. E. Ebert, K. Buehler, and B. Buhler (2010) Redox biocatalysis and metabolism: Molecular mechanisms and metabolic network analysis. Antioxid. Redox Signal. 13: 349–394.
Munro, A. W., H. M. Girvan, and K. J. McLean (2007) Variations on a (t)heme — novel mechanisms, redox partners and catalytic functions in the cytochrome P450 superfamily. Nat. Prod. Rep. 24: 585–609.
Hannemann, F., A. Bichet, K. M. Ewen, and R. Bernhardt (2007) Cytochrome P450 systems — biological variations of electron transport chains. Biochim. Biophys. Acta-Gen. Subj. 1770: 330–344.
Hilker, B. L., H. Fukushige, C. Hou, and D. Hildebrand (2008) Comparison of Bacillus monooxygenase genes for unique fatty acid production. Prog. Lipid Res. 47: 1–14.
Dietrich, M., S. Eiben, C. Asta, T. A. Do, J. Pleiss, and V. B. Urlacher (2008) Cloning, expression and characterisation of CYP102A7, a self-sufficient P450 monooxygenase from Bacillus licheniformis. Appl. Microbiol. Biotechnol. 79: 931–940.
Lentz, O., V. Urlacher, and R. D. Schmid (2004) Substrate specificity of native and mutated cytochrome P450 (CYP102A3) from Bacillus subtilis. J. Biotechnol. 108: 41–49.
Capdevila, J. H., S. Wei, C. Helvig, J. R. Falck, Y. Belosludtsev, G. Truan, S. E. Graham-Lorence, and J. A. Peterson (1996) The highly stereoselective oxidation of polyunsaturated fatty acids by cytochrome P450 BM-3. J. Biol. Chem. 271: 22663–22671.
Lentz, O., A. Feenstra, T. Habicher, B. Hauer, R. D. Schmid, and V. B. Urlacher (2006) Altering the regioselectivity of cytochrome P450CYP102A3 of Bacillus subtilis by using a new versatile assay system. ChemBioChem. 7: 345–350.
Whitehouse, C. J. C., S. G. Bell, H. G. Tufton, R. J. P. Kenny, L. C. I. Ogilvie, and L. L. Wong (2008) Evolved CYP102A1 (P450(BM3)) variants oxidise a range of non-natural substrates and offer new selectivity options. Chem. Commun. 966–968.
Glieder, A., E. T. Farinas, and F. H. Arnold (2002) Laboratory evolution of a soluble, self-sufficient, highly active alkane hydroxylase. Nat. Biotechnol. 20: 1135–1139.
Peters, M. W., P. Meinhold, A. Glieder, and F. H. Arnold (2003) Regio- and enantioselective alkane hydroxylation with engineered cytochromes P450 BM-3. J. Am. Chem. Soc. 125: 13442–13450.
Park, J. B. (2007) Oxygenase-based whole-cell biocatalysis in organic synthesis. J. Microbiol. Biotechnol. 17: 379–392.
Doo, E. H., W. H. Lee, H. S. Seo, J. H. Seo, and J. B. Park (2009) Productivity of cyclohexanone oxidation of the recombinant Corynebacterium glutamicum expressing chnB of Acinetobacter calcoaceticus. J. Biotechnol. 142: 164–169.
Pandey, B. P., C. Roh, K. Y. Choi, N. Lee, E. J. Kim, S. Ko, T. Kim, H. Yun, and B. G. Kim (2010) Regioselective hydroxylation of daidzein using P450 (CYP105D7) from Streptomyces avermitilis MA4680. Biotechnol. Bioeng. 105: 697–704.
Bae, J. -W., E.-H. Doo, S. -H. Shin, S. -G. Lee, Y. -J. Jeong, J. -B. Park, and S. Park (2009) Development of a recombinant Escherichia coli-based biocatalyst to enable high styrene epoxidation activity with high product yield on energy source. Proc. Biochem. 45: 147–152.
Julsing, M. K., S. Cornelissen, B. Buhler, and A. Schmid (2008) Heme-iron oxygenases: Powerful industrial biocatalysts?. Cur. Opin. Chem. Biol. 12: 177–186.
Rosic, N. N. (2009) Versatile capacity of shuffled cytochrome P450s for dye production. Appl. Microbiol. Biotechnol. 82: 203–210.
Schneider, S., M. G. Wubbolts, D. Sanglard, and B. Witholt (1998) Biocatalyst engineering by assembly of fatty acid transport and oxidation activities for in vivo application of cytochrome P-450(BM-3) monooxygenase. Appl. Environ. Microbiol. 64: 3784–3790.
Ni, Y. and R. R. Chen (2005) Lipoprotein mutation accelerates substrate permeability-limited toluene dioxygenase-catalyzed reaction. Biotechnol. Prog. 21: 799–805.
Riesenberg, D. (1991) High-cell-density cultivation of Escherichia coli. Cur. Opin. Biotechnol. 2: 380–384.
Budde, M., S. C. Maurer, R. D. Schmid, and V. B. Urlacher (2004) Cloning, expression and characterisation of CYP102A2, a self-sufficient P450 monooxygenase from Bacillus subtilis. Appl. Microbiol. Biotechnol. 66: 180–186.
Sambrook, J. and R. W. Russell (2001) Molecular cloning: A laboratory manual. 3rd ed., Cold Spring Harbor Laboratory Press, Cold Spring, NY.
Neidhardt, F. C. (1996) Escherichia coli and Salmonella. ASM Press, Washington D.C.
Vaara, M. and M. Nurminen (1999) Outer membrane permeability barrier in Escherichia coli mutants that are defective in the late acyltransferases of lipid A biosynthesis. Antimicrob. Agents Chemother. 43: 1459–1462.
Vorachek-Warren, M. K., S. Ramirez, R. J. Cotter, and C. R. H. Raetz (2002) A triple mutant of Escherichia coli lacking secondary acyl chains on lipid A. J. Biol. Chem. 277: 14194–14205.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, NR., Yoon, J.W. & Park, JB. Effect of lipopolysaccharide mutation on oxygenation of linoleic acid by recombinant Escherichia coli expressing CYP102A2 of Bacillus subtilis . Biotechnol Bioproc E 16, 7–12 (2011). https://doi.org/10.1007/s12257-010-0243-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-010-0243-4