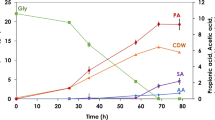
Abstract
In this study, (S)-3-hydroxy-3-phenylpropionate was prepared continuously by coupling microbial transformation and membrane separation. The effect of several factors on membrane flux, reactor capacity, and reaction conversion were investigated. A kinetic model of the continuous reduction process was also developed. The appropriate molecular weight cut-off of the ultrafiltration membrane was 30 kDa. The reactor capacity reached a maximum of 0.136/h at a biomass concentration and membrane flux of 86 g/L (dry weight/reaction volume) and 20 mL/h, respectively. The (S)-3-hydroxy-3-phenylpropionate yield was 3.68 mmol/L/day after continuous reduction over seven days. The enantiometric excess of (S)-3-hydroxy-3-phenylpropionate reached above 99.5%. The kinetic constants of continuous reduction were as follows: r m = 3.00 × 10−3 mol/L/h, k cat = 3.49 × 10−4 mol/L/h, k 1 = 3.09 × 10−2 mol/L, and k 2 = 5.00 × 10−7 mol/L. The kinetic model was in good agreement with the experimental data obtained during continuous reduction. Compared with batch reduction, continuous reduction can significantly improve the catalytic efficiency of microbial cells and increase the reactor capacity.
Similar content being viewed by others
References
Wang, P. Y. and S. W. Tsai (2009) Hydrolytic resolution of (R,S)-3-hydroxy-3-phenylpropionates by esterase from Klebsiella oxytoca: Effects of leaving alcohol, covalent immobilization and aqueous pH. J. Mol. Catal. B: Enzymatic 59: 70–75.
Miles, W. H., E. J. Fialcowitz, and E. Scott Halstead (2001) Enantioselective synthesis of (S)- and (R)-fluoxetine hydrochloride. Tetrahed. 57: 9925–9929.
Hilborn, J. W., Z. H. Lu, A. R. Jurgens, Q. K. Fang, P. Byers, S. A. Walda, and C. H. Senanayake (2001) A practical asymmetric synthesis of (R)-fluoxetine and its major metabolite (R)-norfluoxetine. Tetrahed. Lett. 42: 8919–8921.
Ali, I. S. and A. Sudalai (2002) Pd-catalyzed kinetic resolution of benzylic alcohols: A practical synthesis of (R)-tomoxetine and (S)-fluoxetine hydrochlorides. Tetrahed. Lett. 43: 5435–5436.
He, C. M., D. L. Chang, and J. Zhang (2008) Asymmetric reduction of substituted α- and β-ketoesters by Bacillus pumilus Phe-C3. Tetrahed.: Asymm. 19: 1347–1351.
Lapis, A. A. M., A. De Fatima, J. E. D. Martins, V. E. U. Costa, and R. A. Pilli (2005) Asymmetric reduction of prochiral ketones using in situ generated oxazaborolidine derived from (1S,2S,3R, 4R)-3-amino-7,7-dimethoxynorbornan-2-ol. An efficient synthesis of enantiopure (R)-tomoxetine. Tetrahed. Lett. 46: 495–498.
Nesterov, V. V. and O. I. Kolodiazhnyi (2007) Efficient method for the asymmetric reduction of α- and β-ketophosphonates. Tetrahed. 63: 6720–6731.
Matsumae, H., H. Douno, S. Yamada, T. Nishida, Y. Ozaki, T. Shibatani, and T. Tosa (1995) Microbial asymmetric reduction of (RS)-2-(4-Methoxyphenyl)-1,5-benzothiazepin-3,4(2N,SH)-dione, the key intermediatein the synthesis of diltiazem hydrochloride. J. Ferment. Bioeng. 79: 28–32.
Lou, W. Y., M. H. Zong, Y. Y. Zhang, and H. Wu (2004) Efficient synthesis of optically active organosilyl alcohol via asymmetric reduction of acyl silane with immobilized yeast. Enz. Microbial. Technol. 35: 190–196.
Li, Y. N., X. A. Shi, M. H. Zong, C. Meng, Y. Q. Dong, and Y. H. Guo (2007) Asymmetric reduction of 2-octanone in water/organic solvent biphasic system with Baker’s yeast FD-12. Enz. Microb. Technol. 40: 1305–1311.
Schroer, K., U. Mackfeld, I. A. W. Tan, C. Wandrey, F. Heuser, S. Bringer-Meyer, A. Weckbecker, W. Hummel, T. Daußmann, R. Pfaller, A. Liese, and S. Lütz (2007) Continuous asymmetric ketone reduction processes with recombinant Escherichia coli. J. Biotechnol. 132: 438–444.
Liese, A., T. Zelinski, M. R. Kula, H. Kierkels, M. Karutz, U. Kragl, and C. Wandrey (1998) A novel reactor concept for the enzymatic reduction of poorly soluble ketones. J. Mol. Catal. B: Enzymatic 4: 91–99.
Bubis, M. and N. Zisapel (1998) A role for NAD+ and cADP-ribose in melatonin signal transduction. Mol. Cell. Endocrinol. 137: 59–67.
Antonella, D. C., C. Luca, R. Giulio, B. Francesca, and M. Umberto (2000) Aldose reductase does catalyse the reduction of glyceraldehyde through a stoichiometric oxidation of NADPH. Exp. Eye Res. 71: 515–521.
Ou, Z. M., Z. H. Jin, J. P. Wu, L. R. Yang, and P. L. Cen (2005) Kinetic model of asymmetric reduction of 2′-chloroacetophenone by Saccharomyces cerevisiae B5 with coenzyme regeneration process. J. Chem. Eng. Chin. Univ. 19: 511–517.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ou, Z., Chen, X., Ying, G. et al. Continuous preparation of (S)-3-hydroxy-3-phenylpropionate by asymmetric reduction of 3-oxo-3-phenylpropionic acid ethyl ester with Saccharomyces cerevisiae CGMCC No.2266 in a membrane reactor. Biotechnol Bioproc E 16, 320–326 (2011). https://doi.org/10.1007/s12257-010-0214-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-010-0214-9