Abstract
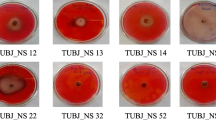
A culture isolated from garden soil was found to be a promising L-glutaminase producer. Biochemical identification tests and 16S rRNA sequencing identified this isolate to be Klebsiella oxytoca. Subsequently, media optimization using one-factor-at-a-time approach and response surface methodology was undertaken. A face centered central composite design was employed to investigate the interactive effects of four variables, viz. concentrations of maltose, yeast extract, beef extract, and ammonium acetate on glutaminase production. Almost all factors had significant interactive effects on glutaminase production. A medium containing (g/L): maltose, 23.31; yeast extract, 20.0; beef extract, 20.01; ammonium acetate, 10.0; mannitol, 10.0; KH2PO4, 0.4; Na2SO4, 0.4; and MgCl2, 0.4 was optimum for glutaminase production. The applied methodology was validated using this optimized media and enzyme activity of 458.91 ± 9.49 U/L and specific activity of 0.441 ± 0.04 U/mg protein after 42 h of incubation at 33°C were obtained.
Similar content being viewed by others
References
Rajeev Kumar, S. and M. Chandrasekaran (2003) Continuous production of L-glutaminase by an immobilized marine Pseudomonas sp BTMS-51 in a packed bed reactor. Proc. Biochem. 38: 1431–1436.
Nagendra Prabhu, G. and M. Chandrasekaran (1997) Impact of process parameters on L-glutaminase production by marine Vibrio costicola in solid state fermentation using polystyrene as an inert support. Proc. Biochem. 32: 285–289.
Ogawa, J. and S. Shimizu (1999) Microbial enzymes: New industrial applications from traditional screening methods. TIBTECH 17: 13–20.
Clarkson, K., B. Jones, R. Bott, B. Bower, G. Chotani, and T. Becker (2001) Enzymes: Screening, Expression, Design and Production. pp 315–352. In: M. R. Bedford and G. G. Partridge (eds.). Enzymes in Farm Animal Nutrition. CAB International, Oxon, UK.
Li, X. and L. Qin (2005) Metagenomics-based drug discovery and marine microbial diversity. TRENDS Biotechnol. 23: 539–543.
Greasham, R. L. (1983) Bioprocessing. Vol. 3, pp. 128–139. In: Rehm, H. J., G. Read, A. Puhler, and P. Stagler (eds.). Biotechnology. VCH Publishers Inc., NY.
Cochran, W. G. and G. M. Cox (1992) Experimental Designs. 4th Ed. Wiley, NY.
Cavalitto, S. F and C. F. Mignone (2007) Application of factorial and Doehlert designs for optimization of protopectinase production by a Geotrichum klebahnii strain. Proc. Biochem. 42: 175–179.
Kalil, S. J., F. Maugeri, and M. I. Rodrigues (2000) Response surface analysis and simulation as a tool for bioprocess design and optimization. Proc. Biochem. 35: 539–550.
Kulikova, A. I. and Iu. V. Galaev (1982) Metabolism of dicarboxylic amino acids and their amides in bacteria of the genus Klebsiella. Zh. Mikrobiol. Epidemiol. Immunobiol. 6: 44–47.
Moriguchi, M., K. Sakai, R. Tateyama, Y. Furuta, and M. Wakayama (1994) Isolation and characterization of salt-tolerant glutaminases from marine Micrococcus luteus K-3. J. Ferment. Bioeng. 77: 621–625.
Iyer, P. and R. Singhal (2008) Production of glutaminase (E.C.3.2.1.5) from Zygosaccharomyces rouxii: Statistical optimization using response surface methodology. Bioresour. Technol. 99: 4300–4307.
Curthoys, N. P. and R. F. Weiss (1974) Regulation of renal ammoniagenesis — subcellular localization of rat kidney glutaminase isoenzymes. J. Biol. Chem. 249: 3261–3266.
Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275.
Tamer, M. and Y. Chisti (2001) Production and recovery of recombinant protease inhibitor α1-antitrypsin. Enz. Microbiol. Technol. 29: 611–620.
Roberts, J., J. S. Holcenberg, and W. C. Dolowy (1972) Isolation, crystallization and properties of Achromobacteraceae glutaminase-asparaginase with antitumor activity. J. Biol. Chem. 247: 84–90.
Omura, H., F. Sato, and K. Hayano (1983) A method for estimating L-glutaminase activity in soils. Soil Sci. P1 Nutr. (Tokyo) 29: 295–303.
Dharmaraj, K., N. Selvakumar, D. Chandramohan, and R. Natarajan (1977) L-glutaminase activity in marine sediments. Ind. J. Mar. Sci. 6: 168–170.
Galstyan, A. and E. G. Saakyan (1973) Determination of soil Lglutaminase activity. Dolk Akad Nauk SSSR 209: 1201–1202.
Santos, V. L., E. F. Araujo, E. G. Barros, and W. V. Guimaraes (1999) Fermentation of starch by Klebsiella oxytoca P2 containing plasmids with α-amylase and pullulanase genes. Biotech. Bioeng. 65: 673–676.
Cole, J. R., B. Chai, R. J. Farris, Q. Wang, A. S. Kulam-Syed-Mohideen, D. M. McGarrell, A. M. Bandela, E. Cardenas, G. M. Garrity, and J. M. Tiedje (2007) The ribosomal database project (RDP-II): introducing myRDP space and quality controlled public data. Nucleic Acids Res. 35(Database issue): D169–D172.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Padma, I., Singhal, R. Isolation, screening, and selection of an L-glutaminase producer from soil and media optimization using a statistical approach. Biotechnol Bioproc E 15, 975–983 (2010). https://doi.org/10.1007/s12257-009-0187-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-009-0187-8