Abstract
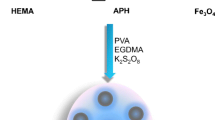
A new metal-chelate adsorbent utilizing 2-methacryloylamidohistidine (MAH) was prepared as a metalchelating ligand. MAH was synthesized using methacryloly chloride and histidine. Monosize nanospheres with an average diameter of 450 nm were produced by emulsion polymerization of 2-hydroxyetylmethacrylate (HEMA) and MAH. Then, Fe3+ ions were chelated directly onto the monosize nanospheres. Mon-poly(HEMA-MAH) nanospheres were characterized by Fourier transform infrared spectroscopy, scanning electron microscopy, and elemental analysis. Fe3+ chelated monosize nanospheres were used in ferritin adsorption from an aqueous solution. The maximum ferritin adsorption capacity of Fe3+-chelated mon-poly(HEMAMAH) nanospheres was 202 mg/g at pH 4.0 in acetate buffer. The non-specific ferritin adsorption on the monpoly( HEMA-MAH) nanospheres was 20 mg/g. The adsorption behavior of ferritin could be modeled using both Langmuir and Freundlich isotherms. The adsorption capacity decreased with increasing ionic strength of the binding buffer. High desorption ratios (> 95% of the adsorbed ferritin) were achieved with 1.0 M NaCl at pH 7.0. Ferritin could be repeatedly adsorbed and desorbed with the Fe3+-chelated mon-poly(HEMA-MAH) nanospheres without significant loss of adsorption capacity.
Similar content being viewed by others
References
Safarik, I. and M. Safarikova (2004) Magnetic techniques for the isolation and purification of proteins and peptides. Biomagnetic Res. Technol. 2: 7.
Karataş, M., S. Akgöl, H. Yavuz, R. Say, and A. Denizli (2007) Immunoglobulin G depletion from human serum with metal-chelated beads under magnetic field. Int. J. Biol. Macromol. 40: 254–260.
Porath, J., J. Carlsson, I. Olsson, and G. Belfrage (1975) Metal chelate affinity chromatography, a new approach to protein fractionation. Nature 258: 598–599.
Gupta, M. N., S. Jain, and I. Roy (2002) Immobilized metal affinity chromatography without chelating ligands: Purification of soybean trypsin inhibitor on Zinc alginate beads. Biotechnol. Prog. 18: 78–81.
Ivanov, A. E., I. Y. Galaev, S. V. Kazakov, and B. Mattiasson (2001) Thermosensitive copolymers of N-vinylimidazole as displacers of proteins in immobilised metal affinity chromatography. J. Chromatography A. 907: 115–130.
Abudiab, T. and R. R. Beitle (1998) Preparation of magnetic immobilized metal affinity separation media and its use in the isolation of proteins. J. Chromatography A. 795: 211–217.
Kim, Y. J. (1999) Optimized operating parameters for the displacement separation of biomolecules in immobilized metal ion affinity chromatography. Biotechnol. Techniq. 13: 837–842.
Akgöl, S., N. ztürk, and A. Denizli (2008) Dye affinity hollow fibers for β-casein. React. Function. Polym. 68: 225–232.
Akgöl, S., N. Bereli, and A. Denizli (2005) Magnetic dye affinity beads for the adsorption of beta-casein. Macromol. Biosci. 5: 786–794.
Çanak Y., S. Özkara, S. Akgöl, and A. Denizli (2004) Pseudospecific biochromatography of immunoglobulin G. React. Function. Polym. 61: 369–377.
Yavuz, H., M. Odaba, S. Akgöl, and A. Denizli (2005) Immobilized metal affinity beads for ferritin adsorption. J. Biomat. Sci. Polym. Edition. 16: 673–684.
Sarı, M., A. Akgöl, M. Karataı, and A. Denizli (2006) Reversible immobilization of catalase by metal chelate affinity interaction on magnetic beads. Ind. Eng. Chem. Res. 45: 3036–3043.
Akgöl, S., N. Öztürk, A. A. Karagözler, D. A. Uygun, M. Uygun, and A. Denizli (2008) A new metal-chelated beads for reversible use in uricase adsorption. J. Mol. Catal. B: Enzy. 51: 36–41.
Porath, J. (1992) Immobilized metal ion affinity chromatography. Protein Express. Purif. 3: 263–281.
Ueda, E. K. M., P. W. Gout, and L. Morganti (2003) Current and prospective applications of metal ion-protein binding. J. Chromatography A. 988: 1–23.
Gaberc-Porekar, V. and V. Menart (2005) Potential for using histidine tags in purification of proteins at large scale. Chem. Eng. Technol. 28: 1306–1314.
Ribeiro, M. B., M. Vijayalakshmi, D. Todorova-Balvay, and S. M. A. Bueno (2008) Effect of IDA and TREN chelating agents and buffer systems on the purification of human IgG with immobilized nickel affinity membranes. J. Chromatograp. B. 861: 64–73.
David, A. E., N. S. Wang, V. C. Yang, and A. J. Yang (2006) Chemically surface modified gel (CSMG): An excellent enzymeimmobilization matrix for industrial processes. J. Biotechnol. 125: 395–407.
Akgöl, S., N. Tüzmen and A. Denizli (2007) Porous dye affinity beads for albumin separation from human plasma. J. Appl. Polym. Sci. 105: 1251–1260.
Wu, J., M. Luan, and J. Zhao (2006) Trypsin immobilization by direct adsorption on metal ion chelated macroporous chitosan-silica gel beads. Int.J. Biol.l Macromol. 39: 185–191.
Öztürk, N., M. Arýsoy S. Akgöl, and A. Denizli (2007) Reversible adsorption of lipase on novel hydrophobic nanospheres. Sep. Purif. Technol. 58: 83–90.
San-Marina, S. and D. M. Nicholls (1995) Metal binding and ferritin immunoreactivity in a high molecular weight fraction from rat brain. Biochimica et Biophysica Acta (BBA) — Mol. Cell Res. 1310: 277–283.
Theil, E. C. (1987) Ferritin: Structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu. Rev. Biochem. 56: 289–315.
Hynes, M. J. and M. O’Coinceanainn (2004) The kinetics and mechanisms of reactions of iron(III) with caffeic acid, chlorogenic acid, sinapic acid, ferulic acid and naringin. J. Inorganic Biochem. 98: 1457–1464.
Caruso, F., D. N. Furlong, and P. Kingshott (1997) Characterization of ferritin adsorption onto gold. J. ColloidInterf. Sci. 186: 129–140.
Bradford, M. M. (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
Rauf, M. A., S. B. Bukallah, F. A. Hamour, and A. S. Nasir (2007) Adsorption of dyes from aqueous solutions onto sand and their kinetic behavior. Chem. Eng. J. 137: 238–243.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uygun, D.A., Öztürk, N., Akgöl, S. et al. Methacryloylamidohistidine in affinity ligands for immobilized metal-ion affinity chromatography of ferritin. Biotechnol Bioproc E 16, 173–179 (2011). https://doi.org/10.1007/s12257-009-0162-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-009-0162-4