Abstract
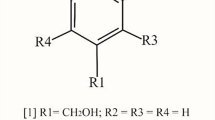
A rapid method for the determination of fungal glucosamine (GlcN) from Aspergillus sp BCRC 31742 was developed. The hydrochlorination process using microwave effectively reduced reaction time needed for GlcN analysis. The analytical method consisted of two steps: (1) hydrochlorination of fungal cells and (2) derivatization process. Fungal GlcN hydrochloride was reacted with 1-napthyl isothiocyanate (1-NITC) as the derivatizing agent to enhance the sensitivity of GlcN and so to achieve high resolution. This method was specific for quantification of GlcN hydrochloride at the wavelength of 230 nm. The standard deviation and relative error of the analytical results were less than 5%. By using microwave heating, the reaction time of hydrochlorination process was shortened from 24 h to 3 min. Thus, the overall time needed for analyzing GlcN from fungal sources was reduced from 5 h (thermal method) to 2 h (microwave method).
Similar content being viewed by others
References
Hsieh, J. W., H. S. Wu, Y. H. Wei, and S. S. Wang (2007) Determination and kinetics of producing glucosamine using fungi. Biotechnol. Prog. 23: 1009–1016.
Nwe, N. and W. F. Stevens (2004) Effect of urea on fungal chitosan production in solid substrate fermentation. Process Biochem. 39: 1639–1642.
Nwe, N. and W. F. Stevens (2002) Production of fungal chitosan by solid substrate fermentation followed by enzymatic extraction. Biotechnol. Lett. 24: 131–134.
Chen, W. and R. Y. Y. Chiou (1999) A modified chemical procedure for rapid determination of glucosamine and its application for estimation of mold growth in peanut kernels and koji. J. Agric. Food Chem. 47: 1999–2004.
Kim, W. J., W. G. Lee, K. Theodore, and H. N. Chang (2001) Optimization of culture conditions and continuous production of chitosan by the fungi, Absidia coerulea. Biotechnol. Bioprocess Eng. 6: 6–10.
Sumbali, G. (2005) The fungi. 1st ed., pp. 230–250. Alpha Science International Ltd, UK.
Mojarrad, J. S., M. Nemati, H. Valizadeh, M. Ansarin, and S. Bourbour (2007) Preparation of glucosamine from exoskeleton of shrimp and predicting production yield by response surface methodology. J. Agric. Food Chem. 55: 2246–2250.
Anderson, J. W., R. J. Nicolosi, and J. F. Borzelleca (2005) Glucosamine effects in humans: a review of effects on glucose metabolism, side effects, safety considerations, and efficacy. Food Chem. Toxicol. 43: 187–201.
Jung, W. J., G. H. Jo, J. H. Kuk, K. Y. Kim, and R. D. Park (2005) Demineralization of crab shells by chemical and biological treatments. Biotechnol. Bioprocess Eng. 10: 67–72.
Yu, K. W., Y. S. Kim, K. S. Shin, J. M. Kim, and H. J. Suh (2005) Macrophage stimulating activity of exobiopolymer from cultured rice bran with Monascus pilosus. Appl. Biochem. Biotechnol. 126: 35–48.
Ruiz-Teran, F. and J. D. Owens (1996) Chemical enzymatic changes during the fermentation of bacteria-free soya bean tempe. J. Sci. Food Agric. 71: 523–530.
Carter, S. B., S. E. Nokes, and C. L. Crofcheck (2004) The influence of environmental temperature and substrate initial moisture content on Aspergillus niger growth and phytase production in solid state cultivation. Trans. Americ. Soc. Agric. Eng. 47: 945–949.
Pochanavanich, P. and W. Suntornsuk (2002) Fungal chitosan production and its characterization. Lett. Appl. Microbiol. 35: 17–21.
Chatterjee, S., M. Adhya, A. K. Guha, and B. P. Chatterjee (2005) Chitosan from Mucor rouxii: production and physico-chemical characterization. Process Biochem. 40: 395–400.
Liu, Y., W. Liao, and S. Chen (2008) Co-production of lactic acid and chitin using a pelletized filamentous fungus Rhizopus oryzae cultured on cull potatoes and glucose. J. App. Microbiol. 105: 1521–1528.
Sparringa, R. A. and J. D. Owens (1999) Short communication: Glucosamine content of tempe mould, Rhizopus oligosporus. Int. J. Food Microbiol. 47: 153–157.
Wagner, W. D. (1979) A more sensitive assay discriminating galactosamine and glucosamine in mixtures. Anal. Biochem. 94: 394–396.
Setnikar. I., C. Giachetti, and G. Zanolo (1984) Absorption, distrubition, and excretion of radioactivity after a single intravenous or oral administration of [14C] glucosamine to the rat. Pharmatherapeutica 3: 538–550.
Shinohara, T. (1981) Use of a flame fherminoic detector in the determination of glucosamine and galactosamine in glycoconjugates by gas chromatography. J. Chromatogr. 2: 262–267.
Guttman, A. (1997) Analysis of monosaccharide composition by capillary electophoresis. J. Chromatogr. A. 763: 271–277.
Zamani, A., A. Jeihanipour, L. Edebo, C. Niklasson, and M. J. Taherzadeh (2008) Determination of glucosamine and N-Acetyl glucosamine in fungal cell walls. J. Agric. Food Chem. 56: 8314–8318.
Cai, J., J. Yang, Y. Du, L. Fan, Y. Qiu, J. Li, and J. F. Kennedy (2006) Enzymatic preparation of chitosan from the waste Aspergillus niger mycelium of citric acid production plant. Carbohydr. Polym. 64: 151–157.
Nwe, N., W. F. Stevens, S. Tokura, and H. Tamura (2008) Characterization of chitosan and chitosan-glucan complex extracted from the cell wall of fungus Gongronella butleri USDB 0201 by enzymatic method. Enz. Microb. Technol. 42: 242–251.
White, S. A., P. R. Farina, and I. Fulton (1979) Production and isolation of chitosan from Mucor rouxii. Appl. Environ. Microbiol. 32: 323–328.
Hu, K. J., J. L. Hu, K. P. Ho, and K. W. Yeung (2004) Screening of fungi for chitosan producers, and copper adsorption capacity of fungal chitosan and chitosanaceous materials. Carbohydr. Polym. 58: 45–52.
Hiroshi, M., S. Kaori, W. Kimitsuna, and O. Kazukiyo (1992) Characterization of some fungal chitosans. Biosci. Biotechnol. Biochem. 56: 1901–1905.
Nwe, N., S. Chandrkrachang, W. F. Stevens, T. Maw, T. K Tan, E. Khor, and S. M. Wong (2002) Production of fungal chitosan by solid state and submerged fermentation. Carbohydr. Polym. 49: 235–237.
Zhang, L. J., T. M. Huang, X. L. Fang, X. N. Li, Q. S. Wang, Z. W. Zhang, and X. Y. Sha (2006) Determination of glucosamine sulfate in human plasma by precolumn derivatization using high performance liquid chromatography with fluorescence detection: its application to a bioequivalence study. J. Chromatogr. B. 842: 8–12.
Zhu, X., J. Cai, J. Yang, and Q. Su (2005) Determination of glucosamine in impure chitin samples by highperformance liquid chromatography. Carbohydr. Res. 340: 1732–1738.
Liang, Z., J. Leslie, A. Adebowale, M. Ashraf, and N. D. Eddington (1999) Determination of the nutraceutical, glucosamine hydrochloride, in raw materials, dosage forms, and plasma using pre-column derivatization with ultraviolet HPLC. J. Pharm. Biomed. Anal. 20: 807–814.
Huang, T. M., C. H. Deng, N. Z. Chen, Z. Liu, and G. L. Duan (2006) High performance liquid chromatography for the determination of glucosamine sulfate in human plasma after derivatization with 9-fluorenylmethyl chloroformate. J. Sep. Sci. 29: 2296–2302.
Zhou, J. Z., T. Waszkuc, and F. Mohammed (2005) Determination of glucosamine in raw materials and dietary supplements containing glucosamine sulfate and/or glucosamine hydrochloride by high performance liquid chromatography with FMOC-Su derivatization: collaborative study. J. AOAC. Int. 88: 1048–1058.
Diaz, J., J. L. Lliberia, L. Comellas, and F. B. Puig (1996) Amino acid and amino sugar determination by derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate followed by high-performance liquid chromatography and fluorescence detection. J. Chromatogr. A. 719: 171–179.
Aghazadeh-Habashi, A., S. Sattari, F. Pasutto, and F. Jamali (2002) High performance liquid chromatographic determination of glucosamine in rat plasma. J. Pharm. Pharmaceut. Sci. 5: 176–180.
Polshettiwar, V. and R. S. Varma (2007) Greener and sustainable approaches to the synthesis of pharmaceutically active heterocycles. Curr. Opin. Drug Discovery Dev. 10: 723–737.
Kappe, C. O. (2002) High-speed combinatorial synthesis utilizing microwave irradiation. Curr. Opin. Chem. Biol. 6: 314–320.
Polshettiwar, V. and R. S. Varma (2008) Microwaveassisted organic synthesis and transformations using benign reaction media. Acc. Chem. Res. 41: 629–639.
Zhao, Y. F. and J. Chen (2008) Applications of microwaves in nuclear chemistry and engineering. Pro. Nucl. Energy 50: 1–6.
Bykov, Y. V., K. I. Rybakov, and V. E. Semenov (2001) High-temperature microwave processing of materials. Appl. Phys. 34: 55–75.
Cao, L., Y. Jiang, Y. Yu, X. Wei, and W. Li (2008) Methods for producing glucosamine from microbial biomass. US Patent 0,188,649 A1.
Way, W. K., K. G. Gibson, and A. G. Breite (2000) Determination of glucosamine in nutritional supplements by reversed-phase ion-pairing HPLC. J. Liq. Chromatogr. Rel. Technol. 23: 2861–2871.
Anumula, K. R. and P. B. Taylor (1991) Quantitative determination of phenyl isothiocyanate-derivatized amino sugars and amino sugar alcohols by high-performance liquid chromatography. Anal. Biochem. 197: 113–120.
Altmann, F. (1992) Determination of amino sugars and amino acids in glycoconjugates using precolumn derivatization with o-phthalaldehyde. Anal. Biochem. 204: 215–219.
Hagen, S. R. (1993) High-performance liquid chromatographic quantitation of phenylthiocarbamyl muramic acid and glucosamine from bacterial cell walls. J. Chromatogr. A. 632: 63–68.
Bindlingmeyer, B. A., S. A. Cohen, and T. L. Tarvin (1984) Rapid analysis of amino acids using precolumn derivitization. J. Chromatogr. 336: 93–104.
Spiro, M. J. and R. G. Spiro (1992) Monosaccharide determination of glycoconjugates by reverse phase highperformance liquid chromatography of their phenylthiocarbamyl derivatives. Anal. Biochem. 204: 152–157.
Fu, D. and R. A. O’Neill (1995) Monosaccharide composition analysis of oligosaccharides and glycoproteins by High-Pressure Liquid Chromatography. Anal. Biochem. 227: 377–384.
Roberts, B. A. and C. R. Strauss (2005) Toward rapid, “green”, predictable microwave-assisted synthesis. Acc. Chem. Res. 38: 653–661.
Gabriel, C., S. Gabriel, E. H. Grant, and B. S. J. Halstead (1998) Dielectric parameters relevant to microwave dielectric heating. Chem. Soc. Rev. 27: 213–223.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sitanggang, A.B., Wu, HS. & Wang, S.S. Determination of fungal glucosamine using HPLC with 1-napthyl isothiocyanate derivatization and microwave heating. Biotechnol Bioproc E 14, 819–827 (2009). https://doi.org/10.1007/s12257-009-0105-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-009-0105-0