Abstract
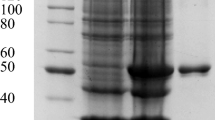
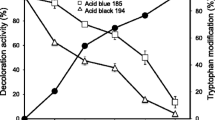
Lignin peroxidase (EC 1.11.1.14) was purified from the Brevibacillus laterosporus MTCC 2298 by ion exchange chromatography. The Km value of the purified lignin peroxidase (using n-propanol as substrate) was 1.6 mM. The MW of purified enzyme determined with the help of MW-standard markers was approximately 205 kDa. Purity of the enzyme was confirmed by native polyacrylamide gel electrophoresis (PAGE) and the activity staining using a substrate L-DOPA. Sulfonated azo dyes such as Methyl orange and Blue-2B were degraded by the purified lignin peroxidase. Degradation of the dyes was confirmed by HPLC, GC-MS, and FTIR spectroscopy. The mainly elected products of Methyl orange were 4-substituted hexanoic acid (m/z = 207), 4-cyclohexenone lactone cation (m/z = 191), and 4-isopropanal-2, 5-cyclohexa-dienone (m/z = 149) and for Blue-2B were 4-(2-hexenoic acid)-2, 5-cyclohexa-diene-one (m/z = 207; M − 1 = 206) and dehydro-acetic acid derivative (m/z = 223).
Similar content being viewed by others
References
Banat, I. M., P. Nigam, D. Singh, and R. Marchant (1996) Microbial decolorization of textile-dye-containing effluents: a review. Bioresour. Technol. 58: 217–227.
Coughlin, M. F., B. K. Kinkle, and P. L. Bishop (1999) Degradation of azo dyes containing aminonaphthol by Sphingomonas sp. strain 1CX. J. Ind. Microbiol. Biotechnol. 23: 341–346.
Nachiyar, C. V. and G. S. Rajkumar (2003) Degradation of a tannery and textile dye, Navitan Fast Blue S5R by Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 19: 609–614.
Dua, M., A. Singh, N. Sethunathan, and A. K. Johri (2002) Biotechnology and bioremediation: successes and limitations. Appl. Microbiol. Biotechnol. 59: 143–152.
Huang, Q., J. Walter, and J. R. Weber (2002) Peroxidase-catalyzed oxidative coupling of phenols in the presence of geosorbents: Effects of sorbent chemical characteristics, American Chemical Society, Environmental Chemistry Awards Symposia, 224th ACS National Meeting, Boston, MA, USA.
Pasti-Grigsby, M. B., A. Paszczynski, S. Goszczynski, D. L. Crawford, and R. L. Crawford (1992) Influence of aromatic substitution patterns on azo dye degradability by Streptomyces spp. and Phanerochaete chrysosporium. Appl. Environ. Microbiol. 58: 3605–3613.
Martinez, A. T., M. Speranza, F. J. Ruiz-Duenas, P. Ferreira, S. Camarero, F. Guillen, M. J. Martinez, A. Gutierrez, and J. C. del Rio (2005) Biodegradation of lignocellulosics: microbial, chemical, and enzymatic aspects of the fungal attack of lignin. Int. Microbiol. 8: 195–204.
Linke, D., H. Zorn, B. Gerken, H. Parlar, and R. G. Berger (2007) Laccase isolation by foam fractionation: New prospects of an old process. Enzyme Microb. Technol. 40: 273–277.
Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275.
Shanmugam, V., M. Kumari, and K. D. Yadav (1999) n-Propanol as a substrate for assaying the ligninperoxidase activity of Phanerochaete chrysosporium. Indian J. Biochem. Biophys. 36: 39–43.
Ramachandra, M., D. L. Crawford, and G. Hertel (1988) Characterization of an extracellular lignin peroxidase of the lignocellulolytic actinomycete Streptomyces viridosporus. Appl. Environ. Microbiol. 54: 3057–3063.
Hossain, S. M. and N. Anantharaman (2006) Activity enhancement of ligninolytic enzymes of Trametes versi-color with bagasse powder. Afr. J. Biotechnol. 5: 189–194.
Tuisel, H., R. Sinclair, J. A. Bumpus, W. Ashbaugh, B. J. Brock, and S. D. Aust (1990) Lignin peroxidase H2 from Phanerochaete chrysosporium: purification, characterization and stability to temperature and pH. Arch. Biochem. Biophys. 279: 158–166.
Fakoussa, R. M. and M. Hofrichter (1999) Biotechnology and microbiology of coal degradation. Appl. Microbiol. Biotechnol. 52: 25–40.
Kumari, M., R. S. Yadav, and K. D. Yadav (2002) Secretion of ligninperoxidase by Penicillium citrinum, Fusarium oxysporum and Aspergillus terreus. Indian J. Exp. Biol. 40: 802–806.
Tien, M., T. K. Kirk, C. Bull, and J. A. Fee (1986) Steady-state and transient-state kinetic studies on the oxidation of 3,4-dimethoxybenzyl alcohol catalyzed by ligninase of Phanerochaete chrysosporium burds. J. Biol. Chem. 261: 1687–1693.
Pclczar, M. J., E. C. S. Chen, and N. R. Kring (2004) Microbiology. 5th ed., Tata McGraw-Hill Publishing Co. Ltd., New Delhi, India.
Tien, M. and T. K. Kirk (1984) Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H2O2-requiring oxygenase. Proc. Natl. Acad. Sci. USA 81: 2280–2284.
Andrawis, A., K. A. Johnson, and M. Tien (1988) Studies on compound I formation of the lignin peroxidase from Phanerochaete chrysosporium. J. Biol. Chem. 263: 1195–1198.
Hu, Z. C., R. A. Korus, C. R. Venkataramu, and R. L. Crawford (1993) Deactivation kinetics of lignin peroxidase from Phanerochaete chrysosporium. Enzyme Microb. Technol. 15: 567–574.
Yang, J. S., H. L. Yuan, H. X. Wang, and W. X. Chen (2005) Purification and characterization of lignin peroxidases from Penicillium decumbens P6. World J. Microbiol. Biotechnol. 21: 435–440.
Ward, G., Y. Hadar, I. Bilkis, and C. G. Dosoretz (2003) Mechanistic features of lignin peroxidase-catalyzed oxidation of substituted phenols and 1,2-dimethoxyarenes. J. Biol. Chem. 278: 39726–39734.
Ruiz-Duenas, F. J., S. Camarero, M. Perez-Boada, M. J. Martinez, and A. T. Martinez (2001) A new versatile peroxidase from Pleurotus. Biochem. Soc. Trans. 29: 116–122.
Goodwin, D. C., S. D. Aust, and T. A. Grover (1995) Evidence for veratryl alcohol as a redox mediator in lignin peroxidase-catalyzed oxidation. Biochemistry 34: 5060–5065.
Collins, P. J., J. A. Field, P. Teunissen, and A. D. W. Dobson (1997) Stabilization of lignin peroxidases in white rot fungi by tryptophan. Appl. Environ. Microbiol. 63: 2543–2548.
Goszczynski, S., A. Paszczynski, M. B. Pasti-Grigsby, R. L. Crawford, and D. L. Crawford (1994) New pathway for degradation of sulfonated azo dyes by microbial peroxidases of Phanerochaete chrysosporium and Streptomyces chromofuscus. J. Bacteriol. 176: 1339–1347.
Bumpus, J. A. and B. J. Brock (1988) Biodegradation of crystal violet by the white rot fungus Phanerochaete chrysosporium. Appl. Environ. Microbiol. 54: 1143–1150.
Vazquez-Duhalt, R., D. W. S. Westlake, and P. M. Fedorak (1994) Lignin peroxidase oxidation of aromatic compounds in systems containing organic solvents. Appl. Environ. Microbiol. 60: 459–466.
Baciocchi, E., M. F. Gerini, P. J. Harvey, O. Lanzalunga, and S. Mancinelli (2000) Oxidation of aromatic sulfides by lignin peroxidase from Phanerochaete chrysosporium. Eur. J. Biochem. 267: 2705–2710.
Chivukula, M. and V. Renganathan (1995) Phenolic azo dye oxidation by laccase from Pyricularia oryzae. Appl. Environ. Microbiol. 61: 4374–4377.
Reddy, G. V. B., M. D. S. Gelpke, and M. H. Gold (1998) Degradation of 2,4,6-trichlorophenol by Phanerochaete chrysosporium: Involvement of reductive dechlorination. J. Bacteriol. 180: 5159–5164.
Spain, J. C. and D. T. Gibson (1991) Pathway for biodegradation of p-nitrophenol in a Moraxella sp. Appl. Environ. Microbiol. 57: 812–819.
Martins, M. A. M., I. C. Ferreira, I. M. Santos, M. J. Queiroz, and N. Lima (2001) Biodegradation of bioaccessible textile azo dyes by Phanerochaete chrysosporium. J. Biotechnol. 89: 91–98.
Soares, G. M. B., M. T. P. Amorim, R. Hrdina, and M. Costa-Ferreira (2002) Studies on the biotransformation of novel disazo dyes by laccase. Process Biochem. 37: 581–587.
Baciocchi, E., M. F. Gerini, O. Lanzalunga, A. Lapi, S. Mancinelli, and P. Mencarelli (2000) Oxidative N-demethylation of N,N-dimethylanilines catalyzed by lignin peroxidase: a mechanistic insight by a kinetic deuterium isotope effect study. Chem. Commun. 5: 393–394.
Baciocchi, E., M. F. Gerini, O. Lanzalunga, A. Lapi, M. G. Lo Piparo, and S. Mancinelli (2001) Isotope-effect profiles in the oxidative N-demethylation of N,N-dimethylanilines catalyzed by lignin peroxidase and a chemical model. Eur. J. Org. Chem. 12: 2305–2310.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gomare, S.S., Jadhav, J.P. & Govindwar, S.P. Degradation of sulfonated azo dyes by the purified lignin peroxidase from Brevibacillus laterosporus MTCC 2298. Biotechnol Bioproc E 13, 136–143 (2008). https://doi.org/10.1007/s12257-008-0008-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-008-0008-5