Summary
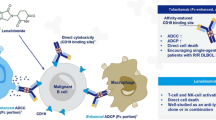
The treatment landscape of aggressive B cell lymphomas has changed substantially in recent years. Several therapeutic agents changed the dogma of diffuse large B cell lymphoma (DLBCL) as a one shot cancer. CD-19-targeted CART cell therapy, CD79b-targeted antibody drug conjugate polatuzumab vedotin, and CD 19 antibody tafasitamab in combination with lenalidomide are approved in relapsed/refractory (r/r) disease. Recently the bispecific CD20/CD3 antibody glofitamab received approval for third-line therapy and approval for other CD20/CD3 bispecific antibodies is expected soon. This short review is a personal selection of the clinically most relevant abstracts besides CART therapy regarding aggressive B cell lymphomas presented at the 2022 ASH meeting in San Diego.
Similar content being viewed by others
References
Illerhaus G et al. Effects on survival of non-myeloablative chemoimmunotherapy compared to high-dose chemotherapy followed by autologous stem cell transplantation (HDC-ASCT) as consolidation therapy in patients with primary cns lymphoma - results of an international randomized phase III trial (MATRix/IELSG43). Blood. 2022;140(suppl 2):LBA-3.
Topp MS, et al. Glofitamab plus R‑CHOP induces high response rates and a favorable safety profile in patients with previously untreated diffuse large B‑cell lymphoma (DLBCL): results from a phase Ib study. ASH 2022 abstract, Vol. 777. 2022.
Abrisqueta P, et al. Subcutaneous Epcoritamab + R‑Dhax/C in patients with relapsed or refractory diffuse large B cell lymphoma eligible for autologous stem cell transplant: updated phase 1/2 results. ASH 2022 abstract, Vol. 443. 2022.
Won-Seog K, et al. Odronextamab in patients with relapsed/refractory (R/R) diffuse large B‑cell lymphoma (DLBCL): results from a prespecified analysis of the pivotal phase II study ELM‑2. ASH 2022 Abtract, Vol. 444. 2022.
Dickinson MJ, et al. Glofitamab for relapsed or refractory diffuse large B‑cell lymphoma. N Engl J Med. 2022;387(24):2220–31.
Hutchings M, et al. Relapse is uncommon in patients with large B‑cell lymphoma who are in complete remission at the end of fixed-course Glofitamab treatment. ASH 2022 Abstract, Vol. 441. 2022.
Kater AP, et al. Subcutaneous Epcoritamab in patients with richter’s syndrome: early results from phase 1b/2 trial (EPCORE CLL-1). ASH 2022 abstract, Vol. 348. 2022.
Duell J, et al. Five-year efficacy and safety of tafasitamab in patients with relapsed or refractory DLBCL: Final results from the phase II L‑MIND study. AACR 2023 abstract, Vol. CT022. 2023.
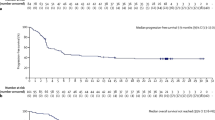
Qualls D, et al. Tafasitamab and Lenalidomide in relapsed/refractory large B cell lymphoma (R/R LBCL): real world outcomes in a multicenter retrospective study. ASH 2022 abstract, Vol. 323. 2022.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Panny has received honoraria from Roche and Abbvie.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Panny, M. Aggressive B cell lymphomas—highlights from ASH 2022. memo 16, 149–151 (2023). https://doi.org/10.1007/s12254-023-00908-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-023-00908-y