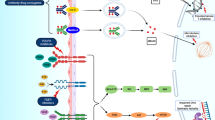
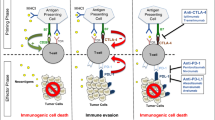
Summary
Single agent immunotherapy is routinely used in the treatment of localised and advanced bladder cancer. Dual checkpoint inhibition with nivolumab (nivo)–ipilimumab (ipi) and durvalumab (durva)–tremelimumab (treme) shows encouraging activity in phase I/II trials in the neoadjuvant and metastatic disease setting. Several large trials are testing these combinations against the current standard of care. The so far reported results are negative; however, it appears that a subgroup of patients might profit substantially from dual checkpoint blockade. Ongoing trials will elucidate which patients will be candidates for this treatment modality in routine clinical care.
Similar content being viewed by others
References
Grimm M‑O, Grün B, Niegisch G, Pichler M, Roghmann F, Schmitz-Dräger B, et al. Tailored immunotherapy approach with nivolumab in advanced transitional cell carcinoma (TITAN-TCC). J Clin Oncol. 2022;40(6_suppl):441–441.
Sharma P, Siefker-Radtke AO, de Braud F, Basso U, Calvo E, Bono P, et al. Nivolumab (N) alone or in combination with ipilimumab (I) in patients (pts) with platinum-pretreated metastatic urothelial carcinoma (mUC): extended follow-up from CheckMate 032. Ann Oncol. 2020;31:S582–S3.
Galsky MD, Powles T, Li S, Hennicken D, Sonpavde G. A phase 3, open-label, randomized study of nivolumab plus ipilimumab or standard of care (SOC) versus SOC alone in patients (pts) with previously untreated unresectable or metastatic urothelial carcinoma (mUC; CheckMate 901). J Clin Oncol. 2018;36(6_suppl):TPS539–TPS539.
Powles T, van der Heijden MS, Castellano D, Galsky MD, Loriot Y, Petrylak DP, et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020;21(12):1574–88.
Galsky MD, Necchi A, Sridhar SS, Ogawa O, Angra N, Hois S, et al. A phase III, randomized, open-label, multicenter, global study of first-line durvalumab plus standard of care (SoC) chemotherapy and durvalumab plus tremelimumab, and SoC chemotherapy versus SoC chemotherapy alone in unresectable locally advanced or metastatic urothelial cancer (NILE). J Clin Oncol. 2021;39(6_suppl):TPS504–TPS504.
van Dijk N, Gil-Jimenez A, Silina K, Hendricksen K, Smit LA, de Feijter JM, et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial. Nat Med. 2020;26(12):1839–44.
Gao J, Siefker-Radtke AO, Navai N, Campbell MT, Slack Tidwell R, Guo C, et al. A pilot presurgical study evaluating anti-PD-L1 durvalumab (durva) plus anti-CTLA‑4 tremelimumab (treme) in patients (pts) with high-risk muscle-invasive bladder carcinoma (MIBC) who are ineligible for cisplatin-based neoadjuvant chemotherapy (NAC). J Clin Oncol. 2019;37(15_suppl):4551–4551.
Grande E, Guerrero F, Puente J, Galante I, Duran I, Dominguez M, et al. DUTRENEO Trial. A randomized phase II trial of DUrvalumab and TREmelimumab versus chemotherapy as a NEOadjuvant approach to muscle-invasive urothelial bladder cancer (MIBC) patients (pts) prospectively selected by an interferon (INF)-gamma immune signature. J Clin Oncol. 2020;38(15_suppl):5012–5012.
Pfister C, Gravis G, Fléchon A, Chevreau C, Mahammedi H, Laguerre B, et al. Dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin or gemcitabine and cisplatin as perioperative chemotherapy for patients with nonmetastatic muscle-invasive bladder cancer: results of the GETUG-AFU V05 VESPER trial. J Clin Oncol. 2022;40(18):2013–22.
Bajorin DF, Witjes JA, Gschwend JE, Schenker M, Valderrama BP, Tomita Y, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021;384(22):2102–14.
Powles T, Drakaki A, Teoh JY‑C, Grande E, Fontes-Sousa M, Porta C, et al. A phase 3, randomized, open-label, multicenter, global study of the efficacy and safety of durvalumab (D) + tremelimumab (T) + enfortumab vedotin (EV) or D + EV for neoadjuvant treatment in cisplatin-ineligible muscle-invasive bladder cancer (MIBC) (VOLGA). J Clin Oncol. 2022;40(6_suppl):TPS579–TPS579.
Garcia del Muro X, Valderrama BP, Medina A, Cuellar MA, Etxaniz O, Gironés Sarrió R, et al. Phase II trial of durvalumab plus tremelimumab with concurrent radiotherapy (RT) in patients (pts) with localized muscle invasive bladder cancer (MIBC) treated with a selective bladder preservation approach: Immunopreserve-Sogug trial. J Clin Oncol. 2021;39(15_suppl):4505–4505.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
K. Mayrhofer and D. Niedersüß-Beke declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mayrhofer, K., Niedersüß-Beke, D. Dual checkpoint-blockade in urothelial carcinoma—when is it so far. memo 16, 52–55 (2023). https://doi.org/10.1007/s12254-022-00839-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-022-00839-0