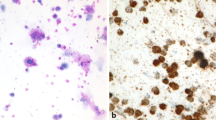
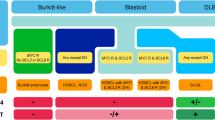
Summary
This short review aims at summarizing the current standards of lymphoma diagnostics and some novelties in the recent WHO classification. The importance of close collaboration between clinicians and pathologists to render the correct diagnosis and to find the most appropriate treatment for each individual patient is highlighted. In lymphomas, the diagnostic evaluation of histopathology, immune phenotype and genetics are puzzle pieces that have to be put into a broader context with the help of the information given by the clinical colleagues, such as patient’s age and sex, location of the lesion, previous medical history and medication. An excision of the affected lymph node is always preferable to fine needle biopsies, as—in many instances—only the evaluation of the whole specimen allows for reliable diagnosis, grading and additional investigations.
The new WHO classification entailed many changes in the category of diffuse large B‑cell lymphoma and high-grade B‑cell lymphoma. The obligatory specification of the cell of origin in diffuse large B‑cell lymphoma is obtained via additional immunohistochemical stainings. The identification of high-grade B‑cell lymphomas with genetic double/triple hits, requiring a more aggressive management, can only be achieved by the detection of chromosomal translocations (MYC, BCL2 and/or BCL6). Significant changes in the classification of T‑cell lymphomas have occurred due to the recognition of the follicular T‑helper cell origin in some instances, and sharpening diagnostic borders of intestinal T‑cell- and Epstein-Barr-virus-associated proliferations. Finally, the discovery of disease-defining and/or prognostically relevant mutations makes the introduction of proper routine molecular testing mandatory.


Similar content being viewed by others
References
Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. US lymphoid malignancy statistics by World Health Organization subtypes. Ca Cancer J Clin. 2016; https://doi.org/10.3322/caac.21357.
Schinasi L, Leon ME. Non-Hodgkin lymphoma and occupational exposure to agricultural pesticide chemical groups and active ingredients: a systematic review and meta-analysis. Int J Environ Res Public Health. 2014;11(4):4449–527.
Tzankov A, Dirnhofer S. A pattern-based approach to reactive lymphadenopathies. Semin Diagn Pathol. 2018;35(1):4–19.
Ansell SM, Armitage JO. Positron emission tomographic scans in lymphoma: convention and controversy. Mayo Clin Proc. 2012;87(6):571–80.
Swerdlow S, Campo E, Harris N, Jaffe E, Pileri S, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC; 2017.
Attarbaschi A, Beishuizen A, Mann G, Rosolen A, Mori T, Uyttebroeck A, et al. Children and adolescents with follicular lymphoma have an excellent prognosis with either limited chemotherapy or with a “watch and wait” strategy after complete resection. Ann Hematol. 2013;92(11):1537–41.
Louissaint A Jr., Schafernak KT, Geyer JT, Kovach AE, Ghandi M, Gratzinger D, et al. Pediatric-type nodal follicular lymphoma: a biologically distinct lymphoma with frequent MAPK pathway mutations. Blood. 2016;128(8):1093–100.
Salaverria I, Philipp C, Oschlies I, Kohler CW, Kreuz M, Szczepanowski M, et al. Translocations activating IRF4 identify a subtype of germinal center-derived B‑cell lymphoma affecting predominantly children and young adults. Blood. 2011;118(1):139–47.
Copie-Bergman C, Niedobitek G, Mangham DC, Selves J, Baloch K, Diss TC, et al. Epstein-Barr virus in B‑cell lymphomas associated with chronic suppurative inflammation. J Pathol. 1997;183(3):287–92.
Cheuk W, Chan AC, Chan JK, Lau GT, Chan VN, Yiu HH. Metallic implant-associated lymphoma: a distinct subgroup of large B‑cell lymphoma related to pyothorax-associated lymphoma? Am J Surg Pathol. 2005;29(6):832–6.
Miranda RN, Aladily TN, Prince HM, Kanagal-Shamanna R, de Jong D, Fayad LE, et al. Breast implant-associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients. J Clin Oncol. 2014;32(2):114–20.
Fong D, Steurer M, Greil R, Gunsilius E, Spizzo G, Gastl G, et al. Hodgkin lymphoma in Tyrol—a population-based study. Ann Hematol. 2009;88(5):449–56.
Menter T, Juskevicius D, Alikian M, Steiger J, Dirnhofer S, Tzankov A, et al. Mutational landscape of B‑cell post-transplant lymphoproliferative disorders. Br J Haematol. 2017;178(1):48–56.
DeStefano CB, Desai SH, Shenoy AG, Catlett JP. Management of post-transplant lymphoproliferative disorders. Br J Haematol. 2018; https://doi.org/10.1111/bjh.15263.
Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B‑cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–11.
Gutiérrez-García G, Cardesa-Salzmann T, Climent F, González-Barca E, Mercadal S, Mate JL, et al. Gene-expression profiling and not immunophenotypic algorithms predicts prognosis in patients with diffuse large B‑cell lymphoma treated with immunochemotherapy. Blood. 2011;117(18):4836–43.
Meyer PN, Fu K, Greiner TC, Smith LM, Delabie J, Gascoyne RD, et al. Immunohistochemical methods for predicting cell of origin and survival in patients with diffuse large B‑cell lymphoma treated with rituximab. J Clin Oncol. 2011;29(2):200–7.
Lunning MA, Vose JM. Angioimmunoblastic T‑cell lymphoma: the many-faced lymphoma. Blood. 2017;129(9):1095–102.
Weiss LM, Chen YY. EBER in situ hybridization for Epstein-Barr virus. Methods Mol Biol. 2013;999:223–30.
Howlett C, Snedecor SJ, Landsburg DJ, Svoboda J, Chong EA, Schuster SJ, et al. Front-line, dose-escalated immunochemotherapy is associated with a significant progression-free survival advantage in patients with double-hit lymphomas: a systematic review and meta-analysis. Br J Haematol. 2015;170(4):504–14.
Aukema SM, Siebert R, Schuuring E, van Imhoff GW, Kluin-Nelemans HC, Boerma EJ, et al. Double-hit B‑cell lymphomas. Blood. 2011;117(8):2319–31.
Hoeller S, Tzankov A, Stenner F, Dirnhofer S. When and how to test for C‑MYC in aggressive B cell lymphomas. J Hematop. 2015;8(1):13–20.
Tzankov A, Xu-Monette ZY, Gerhard M, Visco C, Dirnhofer S, Gisin N, et al. Rearrangements of MYC gene facilitate risk stratification in diffuse large B‑cell lymphoma patients treated with rituximab-CHOP. Mod Pathol. 2014;27(7):958–71.
Swerdlow SH, Kuzu I, Dogan A, Dirnhofer S, Chan JK, Sander B, et al. The many faces of small B cell lymphomas with plasmacytic differentiation and the contribution of MYD88 testing. Virchows Arch. 2016;468(3):259–75.
Wang YL. MYD88 mutations and sensitivity to Ibrutinib therapy. J Mol Diagn. 2018;20(2):264–6.
Turakhia S, Lanigan C, Hamadeh F, Swerdlow SH, Tubbs RR, Cook JR. Immunohistochemistry for BRAF V600E in the differential diagnosis of hairy cell leukemia vs other splenic B‑cell lymphomas. Am J Clin Pathol. 2015;144(1):87–93.
Pettirossi V, Santi A, Imperi E, Russo G, Pucciarini A, Bigerna B, et al. BRAF inhibitors reverse the unique molecular signature and phenotype of hairy cell leukemia and exert potent antileukemic activity. Blood. 2015;125(8):1207–16.
Dohner H, Fischer K, Bentz M, Hansen K, Benner A, Cabot G, et al. p53 gene deletion predicts for poor survival and non-response to therapy with purine analogs in chronic B‑cell leukemias. Blood. 1995;85(6):1580–9.
Buccheri V, Barreto WG, Fogliatto LM, Capra M, Marchiani M, Rocha V. Prognostic and therapeutic stratification in CLL: focus on 17p deletion and p53 mutation. Ann Hematol. 2018;97(12):2269–78.
Nadeu F, Delgado J, Royo C, Baumann T, Stankovic T, Pinyol M, et al. Clinical impact of clonal and subclonal TP53, SF3B1, BIRC3, NOTCH1, and ATM mutations in chronic lymphocytic leukemia. Blood. 2016;127(17):2122–30.
Bottoni A, Rizzotto L, Lai TH, Liu C, Smith LL, Mantel R, et al. Targeting BTK through microRNA in chronic lymphocytic leukemia. Blood. 2016;128(26):3101–12.
Obermann EC, Mueller N, Rufle A, Menter T, Mueller-Garamvoelgyi E, Cathomas G, et al. Clonal relationship of classical Hodgkin lymphoma and its recurrences. Clin Cancer Res. 2011;17(16):5268–74.
Juskevicius D, Lorber T, Gsponer J, Perrina V, Ruiz C, Stenner-Liewen F, et al. Distinct genetic evolution patterns of relapsing diffuse large B‑cell lymphoma revealed by genome-wide copy number aberration and targeted sequencing analysis. Leukemia. 2016;30(12):2385–95.
Montoto S. Treatment of patients with transformed lymphoma. Hematology Am Soc Hematol Educ Program. 2015;2015:625–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M.M. Gerlach and A. Tzankov declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Gerlach, M.M., Tzankov, A. Current lymphoma diagnostic standards: the pathologists’ view. memo 12, 17–23 (2019). https://doi.org/10.1007/s12254-019-0472-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-019-0472-y