Summary
Background
In metastatic colorectal cancer (mCRC), multimodal therapeutic strategies and diagnostics have continuously improved patient survival. The aim of our investigation was to relate this enhanced clinical outcome to treatment costs based on predictive biomarker scenarios guiding epidermal growth factor receptor (EGFR) targeting in a developed country.
Methods
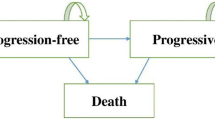
We performed a cost-effectiveness analysis for the combination of EGFR inhibitors with chemotherapy in the first-line treatment of mCRC. Resource use estimates were based on actual data from two oncological departments and on clinical outcomes adapted from published trials. Comparative analyses for the use of EGFR inhibitors were based on three biomarker scenarios (sensitivity: 35%, 55% and 75%) to estimate their incremental cost-effectiveness and were completed by sensitivity analyses.
Results
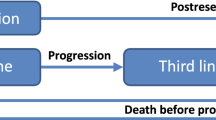
Using FOLFIRI+cetuximab, preselection for EGFR therapy with KRAS testing prolonged progression-free survival with average savings of 913 €/month/patient (scenario 1) and average savings of 1811 €/month/patient when testing the whole RAS-family (scenario 2). In a future but realistic scenario, up 39% of treatment costs could be saved with almost three life–years gained (LYG).
The incremental cost/LYG was 212,083 € (116,646–1,866,332 €) for unselected EGFR therapy, 32,251 € (30,294–43,488 €) for EGFR following KRAS testing, 19,172 € (15,369–28,611 €) for the all RAS scenario, and 12,369 € (3865–18,533 €) for a future biomarker scenario.
Conclusions
In the therapy of mCRC, predictive biomarker testing has shown to be effective and cost saving. For further improvement, a strong research focus on predictive biomarkers is considered highly efficient to promote precision oncology by alleviating the pressure on the healthcare system.

Similar content being viewed by others
References
Cancer research UK. Worldwide cancer statistics. 2017. http://www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer#heading-Zero. Accessed 20 July 2017.
National Cancer Institute. Cancer prevalence and cost of care projects. 2017. https://costprojections.cancer.gov/expenditures.html. Accessed 20 July 2017.
Luengo-Fernandez R, Leal J, Gray A, Sullivan R. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol. 2013;14(12):1165–74.
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–91.
Statistik Austria. 2017. http://www.statistik.at/web_de/statistiken/menschen_und_gesellschaft/gesundheit/krebserkrankungen/dickdarm_enddarm/index.html. Accessed 20 July 2017.
Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27(22):3677–83.
Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–28.
Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(10):1626–34.
Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28(31):4697–705.
Tabernero J, Van Cutsem E, Diaz-Rubio E, Cervantes A, Humblet Y, Andre T, et al. Phase II trial of cetuximab in combination with fluorouracil, leucovorin, and oxaliplatin in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2007;25(33):5225–32.
Van Cutsem E, Kohne CH, Lang I, Folprecht G, Nowacki MP, Cascinu S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29(15):2011–9.
Qiu LX, Mao C, Zhang J, Zhu XD, Liao RY, Xue K, et al. Predictive and prognostic value of KRAS mutations in metastatic colorectal cancer patients treated with cetuximab: a meta-analysis of 22 studies. Eur J Cancer. 2010;46(15):2781–7.
Stintzing S, Jung A, Rossius L, Modest D, Fischer von Weikersthal L, Decker T, et al., editors. Mutations within the EGFR signaling pathway: Influence on efficacy in FIRE-3—A randomized phase III study of FOLFIRI plus cetuximab or bevacizumab as first-line treatment for wild-type (WT) KRAS (exon 2) metastatic colorectal cancer (mCRC) patients. Proceedings of ASCO GI. J Clin Oncol. 2014;32(Suppl 3):445 (abstr).
Kaczirek K, Ciuleanu TE, Vrbanec D, Marton E, Messinger D, Liegl-Atzwanger B, et al. FOLFOX4 plus cetuximab for patients with previously untreated metastatic colorectal cancer according to tumor RAS and BRAF mutation status: updated analysis of the CECOG/CORE 1.2.002 study. Clin Colorectal Cancer. 2015;14(2):91–8.
Bokemeyer C, Kohne CH, Ciardiello F, Lenz HJ, Heinemann V, Klinkhardt U, et al. FOLFOX4 plus cetuximab treatment and RAS mutations in colorectal cancer. Eur J Cancer. 2015;51(10):1243–52.
Lange A, Prenzler A, Frank M, Kirstein M, Vogel A, von der Schulenburg JM. A systematic review of cost-effectiveness of monoclonal antibodies for metastatic colorectal cancer. Eur J Cancer. 2013;50(1):40.
Graham CN, Hechmati G, Hjelmgren J, de Liege F, Lanier J, Knox H, et al. Cost-effectiveness analysis of panitumumab plus mFOLFOX6 compared with bevacizumab plus mFOLFOX6 for first-line treatment of patients with wild-type RAS metastatic colorectal cancer. Eur J Cancer. 2014;50(16):2791–801.
Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065–75.
Venook AP, Niedzwiecki D, Lenz H‑J, Innocenti F, Mahoney MR, O’Neil BH, et al., editors. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC)2014: J Clin Oncol 32:5s, 2014 (suppl; abstr LBA3)
Lenz H, Niedzwiecki D, Innocenti F, editors. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with expanded ras analyses untreated metastatic adenocarcinoma of the colon or rectum (mCRC). ESMO Conference, Abstract 501O; 2014.
BMG. Österreichischer Ernährungsbericht 2012. 2012. http://www.bmg.gv.at/cms/home/attachments/4/5/3/CH1048/CMS1348749794860/oeb12.pdf. Accessed 20 July 2017.
Adam R, De Gramont A, Figueras J, Guthrie A, Kokudo N, Kunstlinger F, et al. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist. 2012;17(10):1225–39.
Nordlinger B, Van Cutsem E, Rougier P, Kohne CH, Ychou M, Sobrero A, et al. Does chemotherapy prior to liver resection increase the potential for cure in patients with metastatic colorectal cancer? A report from the European Colorectal Metastases Treatment Group. Eur J Cancer. 2007;43(14):2037–45.
Van Cutsem E, Nordlinger B, Adam R, Kohne CH, Pozzo C, Poston G, et al. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006;42(14):2212–21.
Jimeno A, Messersmith WA, Hirsch FR, Franklin WA, Eckhardt SG. KRAS mutations and sensitivity to epidermal growth factor receptor inhibitors in colorectal cancer: practical application of patient selection. J Clin Oncol. 2009;27(7):1130–6.
Tol J, Dijkstra JR, Vink-Borger ME, Nagtegaal ID, Punt CJ, Van Krieken JH, et al. High sensitivity of both sequencing and real-time PCR analysis of KRAS mutations in colorectal cancer tissue. J Cell Mol Med. 2009;14(8):2122–31.
Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023–34.
Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–94.
Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347(7):472–80.
Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344(14):1031–7.
Medco Health Solutions. Drug trend report. 2011. http://www.drugtrendreport.com/2011-report. Accessed 10 Sept 2013.
Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883–92.
Russo M, Siravegna G, Blaszkowsky LS, Corti G, Crisafulli G, Ahronian LG, et al. Tumor heterogeneity and lesion-specific response to targeted therapy in colorectal cancer. Cancer Discov. 2016;6(2):147–53.
Siravegna G, Mussolin B, Buscarino M, Corti G, Cassingena A, Crisafulli G, et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med. 2015;21(7):827.
Acknowledgements
The data were partly presented at WCGC (World Congress on Gastrointestinal Cancers) in Barcelona in 2015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
D. Niedersüß-Beke, J. Simon, M. Schiffinger, and R.M. Mader declare that they have no competing interests.
Caption Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Niedersüß-Beke, D., Simon, J., Schiffinger, M. et al. Economic analysis of biomarker-based anti-EGFR therapies in metastatic colorectal cancer in the Austrian context. memo 11, 322–329 (2018). https://doi.org/10.1007/s12254-018-0440-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-018-0440-y