Summary
IO treatments (immuno-oncology treatments) have become reality and are now daily practice or, in some cases, a daily challenge. New recommendations are being made with the prime purpose of increasing alertness and awareness as well as emphasizing standard operating strategies to deal with immune-related adverse events (ir-AEs) in patients treated with immune checkpoint inhibitors (ICI). This brief review refers to systemic reviews, guidelines and meta-analyses, randomized controlled trials and case series published from 2000 to the present. Existing recommendations for optimal management of toxicities vary according to organ systems affected and grading. Grade 1 toxicities (exception to the rule: neurologic, hematologic, cardiac manifestation) require close monitoring. Grade 2 toxicities prompt immediate treatment interruption combined with corticosteroid administration (prednisone or methylprednisolone 0.5–1 mg/kg/day) until the symptoms revert to grade 1 or less. ir-AEs up to grade 3 or 4 justify suspension of treatment together with increased dosage of prednisone or methylprednisolone (1–2 mg/kg/day) combined with close monitoring to continuously adapt the current immunosuppressive strategy. In some cases, a different additional immunosuppressive agent has to be evaluated. Only when all symptoms have disappeared and immunosuppressive treatment produces a response can all immunosuppressive agents be tapered. Endocrinopathies are the exception to the rule and are mostly controllable by hormone replacement, at least in low-grade manifestation. This short review focuses on the main aspects that help manage immune-related side-effects and elucidates all the additional aspects surrounding and contributing to successful treatment and management of cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Immunotherapy and immune-related adverse events
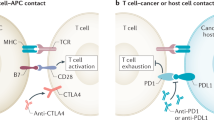
The rise of the checkpoint blockade has been celebrated as the breakthrough of the year 2011. There is a wide diversity of checkpoint-inhibiting antibodies: anti-CTLA-4 (cytotoxic T‑lymphocyte-associated Protein 4; Yervoy® ipilimumab [1]), anti-PD‑1 (programmed cell death Protein 1; Opdivo® nivolumab [2, 3]; Keytruda® pembrolizumab [4]) or anti-PD-L1 directed against the ligand of PD‑1 (programmed death ligand 1; Bavencio® avelumab [5], Tecentriq® atezolizumab [6], Imfinzi® durvalumab [7]), all of which received approval from the US Food and Drug Administration (FDA) and some from the European Medicines Agency (EMA) for treatment of diverse entities of malignancies (metastatic melanoma, metastatic Merkel cell carcinoma, squamous and nonsquamous lung cancer, bladder cancer, metastatic renal cell cancer, therapy-resistant Hodgkin’s lymphoma, head and neck cancer, hepatic cell cancer, mismatch repair-deficient/microsatellite instability-high solid tumors). While these have to be mentioned, the brevity of this overview precludes their discussion.
Treatment of metastatic melanoma and both entities of lung cancer (squamous and nonsquamous cell carcinoma) mainly contributed to the success of ICIs as part of current treatment protocols. While in many cases sustained antitumor response directly correlates with clinical benefit, it may be associated with a wide spectrum of adverse events (presented at the American Association for Cancer Research 2017 Annual Meeting in Washington and commented by Julie Brahmer: Abstract No.: CT077, on 3 April 2017; Checkmate 209-003 ph1b study: 5a OS for the overall study population: 16%, 95% CI 10–23. Survival was seen across PD-L1 expression levels and tumor histologies, but with the bias of not being randomized, blinded or controlled). The reported AEs mainly differ from all known side-effects generated by cytotoxic therapies (chemotherapy, radiotherapy) or antibodies or combinations of them. A wide range of organ systems can be affected (skin, gastrointestinal tract, lungs, endocrine, nervous: peripheral as well as central, ocular, cardiovascular system). The occurring inflammatory reactions strongly resemble autoimmune disorders. While severity is generally mild, even life-threatening complications have been detected. There are some differences between anti-CTLA-4 and anti-PD1/-PDL1 antibodies, especially in grading and duration of occurrence of ir-AEs [8, 9]. There is no direct correlation to drug administration, with infusion related reactions (IRR) documented in very rare cases, namely fewer than 1% of patients. ir-AEs occur even after treatment has been suspended for weeks or even months and this has to be kept in mind when managing patients receiving IO (immuno-oncology) treatment [8].
Recognition is the first step to successful treatment
Many very interesting reviews provide extensive information and elaborate this topic in a concise manner [10,11,12].
Successful management of ir-AEs irrespective of the affected organ systems demands that the patient, family caregivers and the patient’s GP (general practitioner) be kept abreast of the latest IO developments. All of these persons need to be informed about the IO concept and the clinical profile of possible AEs before the concept is initiated, and their information level should be maintained throughout the treatment period. The aim is to generate a “sound” but high level of suspicion for the fact that new symptoms occurring during and even after treatment start may be primarily related to ICI treatment. Grading of toxicities follows CTCAE version 5.0 (National Cancer Institute: Common Terminology Criteria for Adverse Events [CTCAE] 5.0.).
Recommended management of ir-AEs
An increasing number of treatment approaches using ICIs require early recognition of symptoms in connection with changes in laboratory results. Every new symptom must be viewed in connection with the treatment and regarded as an ir-AE until proven otherwise. Diagnostic tools (laboratory results, body scans) are helpful in excluding ir-AEs. Identifying patients at higher risk for ir-AEs demands prompt and aggressive reaction. Recent data suggest that patients suffering from autoimmune diseases are eligible for checkpoint inhibition although they are currently excluded from all trials. There are ways to treat them safely while remaining more alert for unexpected adverse events, although they are still seriously underrepresented [13] and could form a group of high-risk patients. Indeed, such patients do not show a higher incidence of ir-AEs despite having active disease. Hence, treatment-specific risk factors are defined by IO/IO combinations and by the checkpoint inhibitor itself due to differences in incidence and severity of ir-AEs between anti-CTLA-4 [1], anti-PD1 [2,3,4], and anti-PDL1 antibodies [6, 7].
A heightened awareness for early recognition and treatment helps to mitigate the severity of ir-AEs.
Organ-specific recommendations for ir-AEs
In most cases (with some exceptions) ir-AEs grade 2 can be managed by promptly interrupting treatment and providing supportive care. Questionnaires or standardized assessments assist in early recognition. New symptoms or changes in a patient’s health indicate early onset of ir-AEs. Giving patients wallet cards containing detailed information about symptoms and the patient’s immunotherapy history (documented ir-AEs with grading) ensures that all care providers (GPs but also emergency department staff) have additional information to handle the situation and avoid morbidity and ultimately even mortality.
In the case of grade 1 toxicities, watchful continuation of the IO treatment is generally appropriate. However, without complete resolution of symptoms or deterioration to grade 2 or worse immunosuppressive treatment must be started at 0.5 to 1 mg/kg/day prednisone or equivalent corticosteroid combined with tapered discontinuation of treatment. Such tapering of systemic corticosteroids over 4–6 weeks is performed contingent on resolution of the individual’s immune-related reaction. Grade 3 or 4 ir-AEs involve some substantial differences concerning the affected organ system. These differences are discussed in the subsequent part of this article and summarized in Table 1 (pointing out the management of severe adverse events = CTCAE ≥ grade 3 in defined organ toxicities).
Skin toxicities
In the case of failure of high-dose corticosteroid treatment (up to 2 mg/kg/day) an additional immunosuppressive therapy regimen is available: rituximab 375 mg/m2 is recommended instead of high-dose corticosteroids for bullous dermatoses and cyclosporine with or without IVIG (intravenous immunoglobulin) for severe or steroid-resistant cases of SCARs (severe cutaneous adverse reactions) including SJS (Stevens–Johnson syndrome), TEN (toxic epidermal necrolysis), acute generalized exanthematous pustulosis, and DRESS (drug reaction with eosinophilia and systemic syndrome)/DIHS (drug-induced hypersensitivity syndrome).
Enterocolitis
Furthermore, grade 3 or 4 enterocolitis is mainly documented in IO/IO combination approaches (anti-CTLA-4 plus anti-PD-1 antibodies) illustrated in checkmate 063 in advanced melanoma in up to 13% of patients [14]. However, even the sole administration of ICIs results in severe colitis in fewer than <1% of patients, as shown in checkmate 037 and checkmate 066 (both melanoma trials using nivolumab) [15] and checkmate 017 in SQ-NSCLC (nivolumab) [3] and mentioned in treatment with anti-CTLA-4 antibody. This correlates with long-lasting responses persisting even after discontinuation of IO therapy [16]. In the case of grade 3 or 4 ir-AEs early administration of infliximab 5–10 mg/kg must be weighed if there is no response. Therefore, screening laboratory work (like HIV, hepatitis A & B, blood quantiferon for tuberculosis) must be routinely done in patients at high risk in order to be prepared to start infliximab, if and when necessary. Administration of vedolizumab (anti-integrin antibody) is an option in patients who are refractory to infliximab and/or contraindicated for TNF-alpha blockade. Nonsteroidal anti-inflammatory drugs (NSAIDs) are reported to be associated with an increase in ICI-induced enterocolitis [17].
Hepatitis
Hepatitis (grade 3: AST/ALT 5–20 × ULN and/or total bilirubin 3‑10 × ULN) needs a second immunosuppressive agent like mycophenolate mofetil 1 g bid, which should be started if no improvement is assessed after 3 days.
Pneumonitis
Worsening to grade 3 ir-AE always demands permanent discontinuation of ICI, escalated prednisone 1–2 mg/kg/day IV and, if no improvement is observed, the addition of infliximab, MMF or IVIG over 5 days or cyclophosphamide. In life-threatening grade 4 pneumonitis urgent intervention is needed with empirical antibiotics and bronchoscopy with BAL ± transbronchial biopsy if there are any doubts concerning the diagnosis. Less severe pneumonitis (grades 1–2) can be treated with oral prednisone 1–2 mg/kg/day whenever the patient is clinically stable and eligible for regular outpatient visits.
Endocrinopathies
In many cases, endocrine dysfunction (hypothyroidism, hyperthyroidism, adrenal insufficiency, hypophysitis) can be adequately treated on a symptomatic basis except in patients with diabetes, where such treatment could trigger a life-threatening situation as T1DM (type 1 diabetes mellitus) with ketoacidosis demands a strict work-up and insulin substitution.
Cranial MRI (with or without contrast) is indicated for complaints associated with vision changes, severe headaches and multiple endocrine abnormalities (including alterations in diverse electrolytes) in order to exclude hypophysitis [4].
Nervous system toxicities
Neurologic disorders like myasthenia gravis, Guillain–Barré syndrome, peripheral neuropathy, autonomic neuropathy, aseptic meningitis, encephalitis and transverse myelitis are treated with 1–2 mg/kg/day prednisone (grade 3) and, if there is no steroid response, IVIGs or plasmaphereses are ultimately alternative options.
Myocarditis
Cardiovascular complications (myocarditis, pericarditis, arrhythmias, impaired ventricular function with heart failure and vasculitis) require primary administration of corticosteroids. Furthermore, if grading deteriorates (grade 3 or 4) and/or there is no response to corticosteroid, treatment must be adapted by adding mycophenolate mofetil, infliximab or ATG (antithymocyte globulin).
Future aspects and remarks
In the context of ir-AEs, which vary in grading and duration of occurrence, manifestation always correlates with progression-free survival (documented in ongoing and already completed IO studies). Taking into account the fact that responders are primarily long responders, oncologists should maintain a sound awareness for the possibility of evolving immune-related adverse events of diverse grading [10] in order to be able to react promptly and correctly to keep their patients on ICI therapy as long as possible. Therefore, as so often in life, good timing can make all the difference.
References
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23.
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–39.
Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35.
Herbst RS, Baas P, Kim D‑W, Felip E, Perez-Gracia JL, Han J‑Y, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–50.
Palla AR, Doll D. Immunotherapy in Merkel cell carcinoma: role of avelumab. Immunotargets Ther. 2018;7:15–9.
Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–65.
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–29.
Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–7.
Zarogoulidis P, Chinelis P, Athanasiadou A, Tsiouda T, Trakada G, Kallianos A, et al. Possible adverse effects of immunotherapy in non-small cell lung cancer; treatment and follow-up of three cases. Respir Med Case Rep. 2017;22:101–5.
Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018; https://doi.org/10.1200/JCO.2017.77.6385.
Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book. 2015;35:76–83.
Puzanov I, Diab A, Abdallah K, Bingham CO, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95.
Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer. 2017;123:1904–11.
Weber JS, Gibney G, Sullivan RJ, Sosman JA, Slingluff CL, Lawrence DP, et al. Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): an open-label, randomised, phase 2 trial. Lancet Oncol. 2016;17:943–55.
Weber JS, Postow M, Lao CD, Schadendorf D. Management of adverse events following treatment with anti-programmed death-1 agents. Oncologist. 2016;21:1230–40.
Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T‑lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283–9.
Marthey L, Mateus C, Mussini C, Nachury M, Nancey S, Grange F, et al. Cancer immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J Crohns Colitis. 2016;10:395–401.
Funding
Open access funding provided by University of Innsbruck and Medical University of Innsbruck.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J.D. Rudzki declares that he has received honoraria and speaker’s fee from BMS, Roche, MSD, AstraZeneca, Amgen and served as advisor for BMS, Roche, MSD, AstraZeneca, Amgen.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Rudzki, J.D. Management of adverse events related to checkpoint inhibition therapy. memo 11, 132–137 (2018). https://doi.org/10.1007/s12254-018-0416-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-018-0416-y