Abstract
Sarcomas are a rare and heterogeneous group of mesenchymal malignancies that have historically been challenging in diagnosis and treatment. In soft tissue sarcomas (STS), the recognition of different histological subtypes has made a dramatic conceptual change leading to histotype-driven targeted therapies. In gastrointestinal stromal tumors (GIST), the most important advance in the past decade was the demonstration that tyrosine kinase inhibitors (TKIs) can durably control disease and lead to remarkable treatment responses. The advent of TKIs has unquestionably changed the overall survival of patients with GIST, not only in the advanced-disease setting but also in individuals with high-risk disease. This review covers the latest clinical study highlights for STS and GIST presented at American Society of Clinical Oncology (ASCO) 2015 meeting, demonstrating the constant progress from conventional chemotherapy to histology-driven therapy approaches.
Similar content being viewed by others
References
Group E.S.E.S.N.W. Soft tissue and visceral sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii102–12.
Eriksson M. Histology-driven chemotherapy of soft-tissue sarcoma. Ann Oncol. 2010;21 Suppl 7:vii270–6.
Reichardt P. Soft tissue sarcomas, a look into the future: different treatments for different subtypes. Future Oncol. 2014;10(8 Suppl):s19–27.
Group E.S.E.S.N.W. Gastrointestinal stromal tumours: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii21–6.
Blay JY, Reichardt P. Advanced gastrointestinal stromal tumor in Europe: a review of updated treatment recommendations. Expert Rev Anticancer Ther. 2009;9(6):831–8.
Reichardt P, et al. Adjuvant therapy in primary GIST: state-of-the-art. Ann Oncol. 2012;23(11):2776–81.
Tapp WD. A randomized phase Ib/II study evaluating the safety and efficacy of olaratumab (IMC-3G3), a human anti-platelet-derived growth factor α (PDGFRα) monoclonal antibody, with or without doxorubicin (Dox), in advanced soft tissue sarcoma (STS). 2015 ASCO Annual Meeting. 2015.
Mir O, et al. Activity of regorafenib (RE) in leiomyosarcomas (LMS) and other types of soft-tissue sarcomas (OTS): Results of a double-blind, randomized placebo (PL) controlled phase II trial. 2015 ASCO Annual Meeting. 2015.
Kim C, et al. Clinical pattern and implication of PD-L1 expression in soft-tissue sarcoma. ASCO 2015 Annual Meeting. 2015.
Burgess MA. SARC 028: a phase II study of the anti-PD1 antibody pembrolizumab (P) in patients (Pts) with advanced sarcomas. 2015 ASCO Annual Meeting. 2015.
Demetri GD. A randomized phase III study of trabectedin (T) or dacarbazine (D) for the treatment of patients (pts) with advanced liposarcoma (LPS) or leiomyosarcoma (LMS). 2015 ASCO Annual Meeting. 2015.
Seddon BM. GeDDiS: a prospective randomised controlled phase III trial of gemcitabine and docetaxel compared with doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft tissue sarcomas (EudraCT 2009-014907-29). 2015 ASCO Annual Meeting. 2015.
Schoeffski P. Randomized, open-label, multicenter, phase III study of eribulin versus dacarbazine in patients (pts) with leiomyosarcoma (LMS) and adipocytic sarcoma (ADI). 2015 ASCO Annual Meeting. 2015.
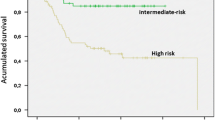
Joensuu H. Three vs. 1 year of adjuvant imatinib (IM) for operable high-risk GIST: The second planned analysis of the randomized SSGXVIII/AIO trial. 2015 ASCO Annual Meeting. 2015.
Chandrajit PR.-. Adjuvant imatinib (IM) for patients (pts) with primary gastrointestinal stromal tumor (GIST) at significant risk of recurrence: PERSIST-5 planned 3-year interim analysis. 2015 ASCO Annual Meeting. 2015.
Blay JY. Final results of the multicenter randomized phase II PAZOGIST trial evaluating the efficacy of pazopanib (P) plus best supportive care (BSC) vs BSC alone in resistant unresectable metastatic and/or locally advanced gastrointestinal stromal tumors (GIST). 2015 ASCO Annual Meeting. 2015.
Chi P. A phase Ib/II study of MEK162 (binimetinib [BINI]) in combination with imatinib in patients with advanced gastrointestinal stromal tumor (GIST). 2015 ASCO Annual Meeting. 2015.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schur, S., Brodowicz, T. Soft tissue sarcomas & GIST Insights into subtype-specific therapy concepts: Highlights from ASCO meeting 2015. memo 8, 205–208 (2015). https://doi.org/10.1007/s12254-015-0233-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-015-0233-5