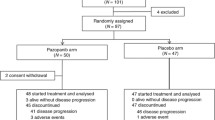
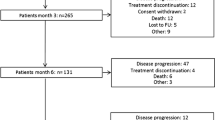
Abstract
The current gold standard of care for first-line therapy in patients with nonsmall cell lung cancer (NSCLC) is a platinum-based doublet chemotherapy, which is recommended for a maximum of six cycles. Since the prolongation of the first-line regimen does not translate into improved survival, and most patients experience progression within 3–4 months after first-line therapy, new treatment strategies have been explored to delay disease progression and prolong survival. Maintenance therapy has been proposed as either continuation maintenance with one part of the induction treatment or as switch maintenance with a different agent not used in first-line therapy. There is an accumulating body of evidence that both strategies administered immediately after induction therapy could improve survival in patients with good performance status. In this review, recent results of pivotal studies are summarized, since some studies have shown impressive benefits in terms of survival and their strategy should be considered as a new treatment option in selected patients.
Similar content being viewed by others
References
Grossi F, Aita M, Follador A, et al. Sequential, alternating, and maintenance/consolidation chemotherapy in advanced non-small cell lung cancer: a review of the literature. Oncologist. 2007;12:451–64.
Gridelli C, Maione P, Rossi A, et al. Potential treatment options after first-line chemotherapy for advanced NSCLC: maintenance treatment or early second-line? Oncologist. 2009;14(2):137–47.
Smith IE, O’Brien MER, Talbot DC, et al. Duration of chemotherapy in advanced non-small-cell lung cancer: a randomized trial of three versus six courses of mitomycin, vinblastine, and cisplatin. J Clin Oncol. 2001;19:1336–43.
Socinski MA, Schell MJ, Peterman A, et al. Phase III trial comparing a defined duration of therapy versus continuous therapy followed by second-line therapy in advanced-stage IIIB/IV non-small-cell lung cancer. J Clin Oncol. 2002;20:1335–43.
von Plessen C, Bergman B, Andresen O, et al. Palliative chemotherapy beyond three courses conveys no survival or consistent quality-of-life benefits in advanced non-small-cell lung cancer. Br J Cancer. 2006;95:966–73.
Park JO, Kim SW, Ahn JS, et al. Phase III trial of two versus four additional cycles in patients who are nonprogressive after two cycles of platinum based chemotherapy in non-small-cell lung cancer. J Clin Oncol. 2007;25:5233–9.
D’Addario G, Früh M, Reck M, et al., ESMO Guidelines Working Group. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v116–9.
Azzoli CG, Temin S, Aliff T, et al., American Society of Clinical Oncology. 2011 focused update of 2009 American Society of Clinical Oncology clinical practice guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol. 2011;29(28):3825–31.
Hensing TA, Schell MJ, Lee JH, Socinski MA. Factors associated with the likelihood of receiving second line therapy for advanced non-small cell lung cancer. Lung Cancer. 2005;47:253–9.
Belani CP, Barstis J, Perry MC, et al. Multicenter, randomized trial for stage IIIB or IV non small cell lung cancer using weekly paclitaxel and carboplatin followed by maintenance weekly paclitaxel or observation. J Clin Oncol. 2003;15:2933–9.
Brodowicz T, Krzakowski M, Zwitter M, et al. Cisplatin and gemcitabine first-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: a phase III trial. Lung Cancer. 2006;52:155–63.
Belani CP, Waterhouse DM, Ghazal H, et al. Phase III study of maintenance gemcitabine (G) and best supportive care (BSC) versus BSC, following standard combination therapy with gemcitabine-carboplatin (G-Cb) for patients with advanced non-small cell lung cancer (NSCLC). J Clin Oncol. 2010;28:15s (suppl; abstr 7506)
Pérol M, Chouaid C, Pérol D, et al. Randomized, phase III study of gemcitabine or erlotinib maintenance therapy versus observation, with predefined second-line treatment, after cisplatin-gemcitabine induction chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2012;30:3516–24.
Zhang X, Zang J, Xu J, et al. Maintenance therapy with continuous or switch strategy in advanced non-small cell lung cancer: a systematic review and meta-analysis. Chest. 2011;140:117–26.
Soon YY, Stockler MR, Askie LM, Boyer MJ. Duration of chemotherapy for advanced non-small-cell lung cancer: a systematic review and meta-analysis of randomized trials. J Clin Oncol. 2009;27(20):3277–83.
Paz-Ares L, De Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012;13:247–55.
Paz-Ares L, De Marinis F, Dediu M, et al. PARAMOUNT: final overall survival (OS) results of the phase III study of maintenance pemetrexed (pem) plus best supportive care (BSC) versus placebo (plb) plus BSC immediately following induction treatment with pem plus cisplatin (cis) for advanced nonsquamous (NS) non-small cell lung cancer (NSCLC). J Clin Oncol. 2012;30 (suppl; abstr LBA7507).
Dansin E, Tsai CM, Pavlakis N, et al., on behalf of the MO19390 (SAiL) Study Group. Safety and efficacy of first-line bevacizumab (bv)-based therapy in advanced non-small cell lung cancer (nsclc): results of the SAIL study (MO 19390) ECCO15/34th ESMO Multidisciplinary Congress Abstract Book. EJC Suppl. 2009;7(2):556 (poster 9168).
Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-smallcell lung cancer. N Engl J Med. 2006;355:2542–50.
Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–34.
Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373:1525–31.
Barlesi F, de Castro J, Dvornichenko V, et al. AVAPERL (MO22089): final efficacy outcomes for patients (pts) with advanced non-squamous non-small cell lung cancer (nsNSCLC) randomised to continuation maintenance (mtc) with bevacizumab (bev) or bev + pemetrexed (pem) after firstline (1L) bev–cisplatin (cis)–pem treatment (Tx). Eur J Cancer. 2011;47:16 (abstr 34LBA).
Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374(9699):1432–40.
Fidias PM, Dakhil SR, Lyss AP, et al. Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:591–8.
Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521–9.
Miller VA, O’Connor P, Soh C, et al., for the ATLAS Investigators. A randomized, double-blind, placebo-controlled, phase IIIb trial (ATLAS) comparing bevacizumab (B) therapy with or without erlotinib (E) after completion of chemotherapy with B for first-line treatment of locally advanced, recurrent, or metastatic non-small cell lung cancer (NSCLC). J Clin Oncol. 2009;27:18s (suppl; abstr LBA8002).
Kabbinavar FF, Miller VA, Johnson BE, et al., on behalf of the ATLAS Investigators. Overall survival (os) in atlas, a phase iiib trial comparing bevacizumab (b) therapy with or without erlotinib (e) after completion of chemotherapy (chemo) with b for first-line t reatment of locally advanced, recurrent, or metastatic non-small cell lung cancer (nsclc). J Clin Oncol. 2010;28:15s (suppl; abstr 7526).
Gaafar RM, Surmont VF, Scagliotti GV, et al., EORTC Lung Cancer Group and the Italian Lung Cancer Project. A double-blind, randomised, placebo-controlled phase III intergroup study of gefitinib in patients with advanced NSCLC, non-progressing after first line platinum-based chemotherapy (EORTC 08021/ILCP 01/03). Eur J Cancer. 2011;47(15):2331–40.
Zhang L, Ma S, Song X, et al., INFORM investigators. Gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer (INFORM; C-TONG 0804): a multicentre, double-blind randomised phase 3 trial. Lancet Oncol. 2012;13(5):466–75.
Conflict of interest
The author declares that there is no actual or potential conflicts of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brodowicz, T. Maintenance therapy in NSCLC. memo 6, 14–18 (2013). https://doi.org/10.1007/s12254-012-0062-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-012-0062-8