Summary
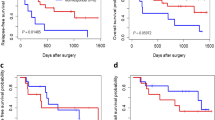
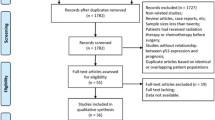
The present review introduces a protocol for a prospective randomized predictive marker clinical trial. The study evaluates the interaction between the proposed predictive marker p53 and response to neoadjuvant chemotherapy in patients with potentially resectable oesophageal cancer. This trial is designed to provide "level of evidence I" data, which are a prerequisite for the clinical implementation of a predictive marker. Despite curative resection, the 5-years survival rates of oesophageal cancer patients do not exceed 20%. Several phase II and III studies have shown that patients who responded to preoperative chemotherapy had a significantly better survival than patients treated with surgery alone. Up till now, it is not possible to identify the responders before commencing treatment. Eighty-four patients with measurable disease will be enrolled in the pANCHO trial. After testing the marker genotype (p53 normal versus p53 mutant), patients will be randomly assigned to receive two different chemotherapies −5FU/Cispaltin or Docetaxel. All patients will be rendered to subsequent surgery and as a primary endpoint response to neoadjuvant treatment will be evaluated. The pANCHO trial is a nationwide academic initiative. The 13 affiliated investigational centres recruited 70% of estimated patients within one year. Patients enrolled in the pANCHO trial benefit from the standardized diagnostic and therapeutic procedures. The study is supposed to identify the need for new guidelines for response assessment in neoadjuvant treated patients. Marker specific results are awaiting autumn 2009 and could be relevant to the choice of therapy in oesophageal cancer.
Similar content being viewed by others
References
Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study – JCOG9204. J Clin Oncol, 21: 4592–4596, 2003
Pouliquen X, Levard H, Hay JM, et al. 5-Fluorouracil and cisplatin therapy after palliative surgical resection of squamous cell carcinoma of the esophagus. A multicenter randomized trial. French Associations for Surgical Research. Ann Surg, 223: 127–133, 1996
Elliot N, Milan P, Theresa R, et al. Neoadjuvant chemotherapy, surgery, and adjuvant intraperitoneal chemotherapy in patients with locally advanced gastric or gastroesophageal junction carcinoma: a phase II study. Seminars in oncology, 32: 97–100, 2005
de Manzoni G, Pedrazzani C, Pasini F, et al. Chemoradiotherapy followed by surgery for squamous cell carcinoma of the thoracic esophagus with clinical evidence of adjacent organ invasion. J Surg Oncol, 95: 261–266, 2007
Korst RJ, Kansler AL, Port JL, et al. Downstaging of T or N predicts long-term survival after preoperative chemotherapy and radical resection for esophageal carcinoma. The Annals of Thoracic Surgery, 82: 480–485, 2006
Makary MA, Kiernan PD, Sheridan MJ, et al. Multimodality treatment for esophageal cancer: the role of surgery and neoadjuvant therapy. Am Surg, 69: 693–700; discussion 700–702, 2003
Urschel JD, Vasan H, A meta–analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg, 185: 538–543, 2003
Vallbohmer D, Lenz HJ, Predictive and prognostic molecular markers in outcome of esophageal cancer. Dis Esophagus, 19: 425–432, 2006
Lowe SW, Ruley HE, Jacks T, et al. p53–dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell, 74: 957–967, 1993
Peller S. Clinical implications of p53: effect on prognosis, tumor progression and chemotherapy response. Semin Cancer Biol, 8: 379–387, 1998
Pirollo KF, Bouker KB, Chang EH. Does p53 status influence tumor response to anticancer therapies? Anticancer Drugs, 11: 419–432, 2000
Wallace–Brodeur RR, Lowe SW. Clinical implications of p53 mutations. Cell Mol Life Sci, 55: 64–75, 1999
Kandioler D. p53 as a prognostic and predictive indicator, in biomarkers in breast cancer. In: Gasparini HF (ed) Molecular diagnostics for predicting and monitoring therapeutic effect. Humana Press: Totowa, pp. 193–209, 2005
Kandioler D, Zwrtek R, Ludwig C, et al. TP53 genotype but not p53 immunohistochemical result predicts response to preoperative short–term radiotherapy in rectal cancer. Ann Surg, 235: 493–498, 2002
Kandioler-Eckersberger D, Kappel S, Mittlbock M, et al. The TP53 genotype but not immunohistochemical result is predictive of response to cisplatin–based neoadjuvant therapy in stage III non-small cell lung cancer. J Thorac Cardiovasc Surg, 117: 744–750, 1999
Kandioler-Eckersberger D, Ludwig C, Rudas M, et al. TP53 mutation and p53 overexpression for prediction of response to neoadjuvant treatment in breast cancer patients. Clin Cancer Res, 6: 50–56, 2000
Sargent DJ, Conley BA, Allegra C, et al. Clinical trial designs for predictive marker validation in cancer treatment trials. J Clin Oncol, 23: 2020–2027, 2005
Kandioler D, Hejna M, Zwrtek R, et al. p53 adapted neoadjuvant therapy for esophageal cancer: Pilot study, p. 206s, 2007 (abstr. 4535)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kandioler, D. Personalized medicine – p53 gene analysis for prediction of response to neoadjuvant therapy in esophageal cancer. memo 1, 137–142 (2008). https://doi.org/10.1007/s12254-008-0049-7
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s12254-008-0049-7